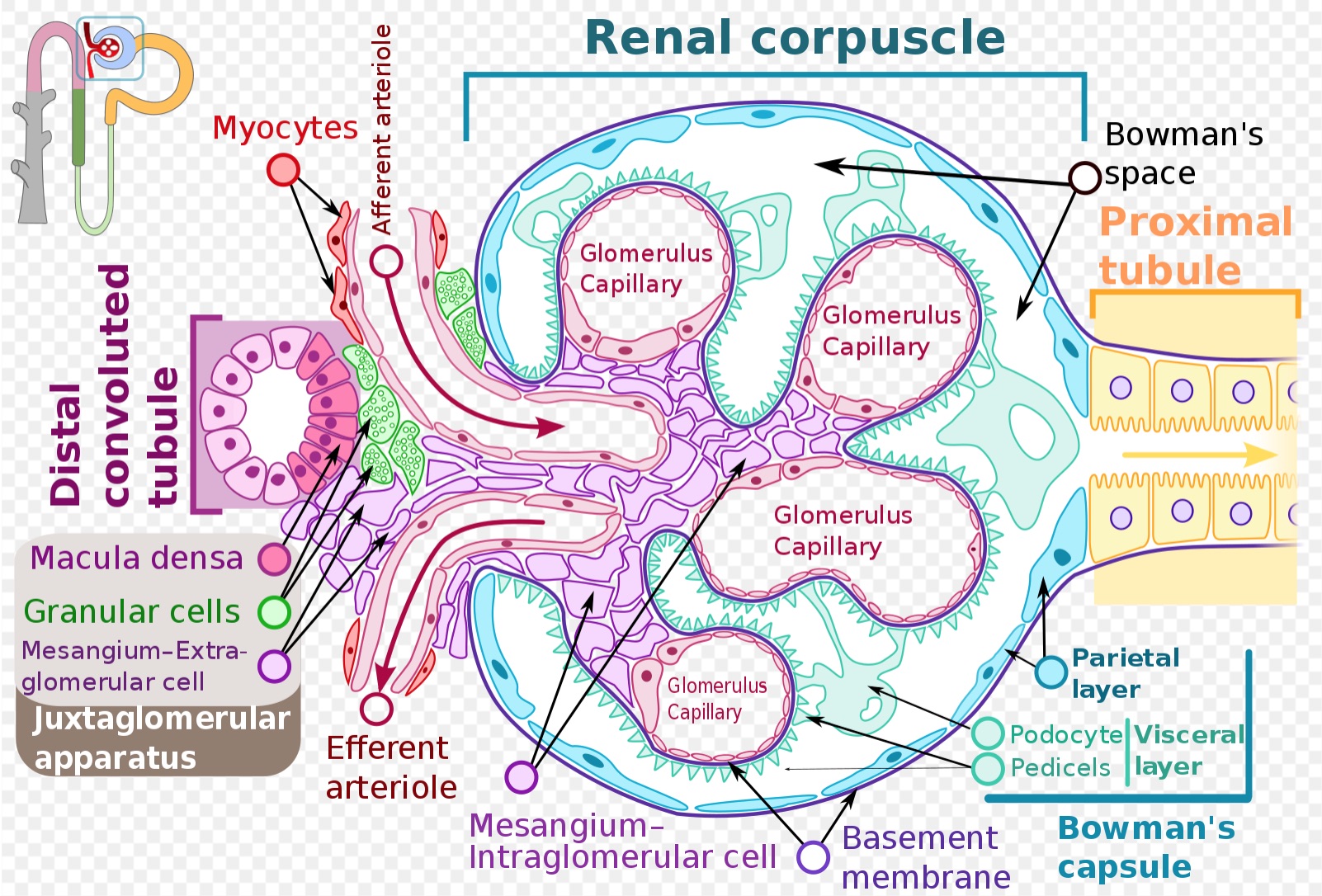
[1]
Puelles VG, Hoy WE, Hughson MD, Diouf B, Douglas-Denton RN, Bertram JF. Glomerular number and size variability and risk for kidney disease. Current opinion in nephrology and hypertension. 2011 Jan:20(1):7-15. doi: 10.1097/MNH.0b013e3283410a7d. Epub
[PubMed PMID: 21099687]
Level 3 (low-level) evidence
[2]
White KE. Research into the structure of the kidney glomerulus--making it count. Micron (Oxford, England : 1993). 2012 Oct:43(10):1001-9. doi: 10.1016/j.micron.2012.04.013. Epub 2012 May 2
[PubMed PMID: 22607953]
[3]
Abrahamson DR. Structure and development of the glomerular capillary wall and basement membrane. The American journal of physiology. 1987 Nov:253(5 Pt 2):F783-94
[PubMed PMID: 3318497]
[4]
Avasthi PS, Evan AP, Hay D. Glomerular endothelial cells in uranyl nitrate-induced acute renal failure in rats. The Journal of clinical investigation. 1980 Jan:65(1):121-7
[PubMed PMID: 7350192]
[5]
Jorgensen F, Bentzon MW. The ultastructure of the normal human glomerulus. Thickness of glomerular basement membrane. Laboratory investigation; a journal of technical methods and pathology. 1968 Jan:18(1):42-8
[PubMed PMID: 5643970]
[6]
Lea PJ, Silverman M, Hegele R, Hollenberg MJ. Tridimensional ultrastructure of glomerular capillary endothelium revealed by high-resolution scanning electron microscopy. Microvascular research. 1989 Nov:38(3):296-308
[PubMed PMID: 2607999]
[7]
Rostgaard J, Qvortrup K. Electron microscopic demonstrations of filamentous molecular sieve plugs in capillary fenestrae. Microvascular research. 1997 Jan:53(1):1-13
[PubMed PMID: 9056471]
[8]
Scott RP, Quaggin SE. Review series: The cell biology of renal filtration. The Journal of cell biology. 2015 Apr 27:209(2):199-210. doi: 10.1083/jcb.201410017. Epub
[PubMed PMID: 25918223]
[9]
Rostgaard J, Qvortrup K. Sieve plugs in fenestrae of glomerular capillaries--site of the filtration barrier? Cells, tissues, organs. 2002:170(2-3):132-8
[PubMed PMID: 11731701]
[10]
Curry FE, Adamson RH. Endothelial glycocalyx: permeability barrier and mechanosensor. Annals of biomedical engineering. 2012 Apr:40(4):828-39. doi: 10.1007/s10439-011-0429-8. Epub 2011 Oct 19
[PubMed PMID: 22009311]
[11]
Zhao JH. Mesangial Cells and Renal Fibrosis. Advances in experimental medicine and biology. 2019:1165():165-194. doi: 10.1007/978-981-13-8871-2_9. Epub
[PubMed PMID: 31399966]
Level 3 (low-level) evidence
[12]
Bussolati B, Bruno S, Grange C, Buttiglieri S, Deregibus MC, Cantino D, Camussi G. Isolation of renal progenitor cells from adult human kidney. The American journal of pathology. 2005 Feb:166(2):545-55
[PubMed PMID: 15681837]
[13]
Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the Bowman's capsule of adult human kidneys. Journal of the American Society of Nephrology : JASN. 2006 Sep:17(9):2443-56
[PubMed PMID: 16885410]
[14]
Ronconi E, Sagrinati C, Angelotti ML, Lazzeri E, Mazzinghi B, Ballerini L, Parente E, Becherucci F, Gacci M, Carini M, Maggi E, Serio M, Vannelli GB, Lasagni L, Romagnani S, Romagnani P. Regeneration of glomerular podocytes by human renal progenitors. Journal of the American Society of Nephrology : JASN. 2009 Feb:20(2):322-32. doi: 10.1681/ASN.2008070709. Epub 2008 Dec 17
[PubMed PMID: 19092120]
[15]
Costantini F, Kopan R. Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Developmental cell. 2010 May 18:18(5):698-712. doi: 10.1016/j.devcel.2010.04.008. Epub
[PubMed PMID: 20493806]
[16]
Yoshimura Y, Nishinakamura R. Podocyte development, disease, and stem cell research. Kidney international. 2019 Nov:96(5):1077-1082. doi: 10.1016/j.kint.2019.04.044. Epub 2019 Jun 3
[PubMed PMID: 31420196]
[17]
Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell stem cell. 2008 Aug 7:3(2):169-81. doi: 10.1016/j.stem.2008.05.020. Epub
[PubMed PMID: 18682239]
[19]
Russell PS, Hong J, Windsor JA, Itkin M, Phillips ARJ. Renal Lymphatics: Anatomy, Physiology, and Clinical Implications. Frontiers in physiology. 2019:10():251. doi: 10.3389/fphys.2019.00251. Epub 2019 Mar 14
[PubMed PMID: 30923503]
[20]
RAWSON AJ. Distribution of the lymphatics of the human kidney as shown in a case of carcinomatous permeation. Archives of pathology. 1949 Mar:47(3):283-92
[PubMed PMID: 18111390]
Level 3 (low-level) evidence
[21]
Bell RD, Keyl MJ, Shrader FR, Jones EW, Henry LP. Renal lymphatics: the internal distribution. Nephron. 1968:5(6):454-63
[PubMed PMID: 5706255]
[22]
Wakiya K, Fukushima T, Miyazaki S. [Results of microvascular decompression in 16 cases of glossopharyngeal neuralgia]. Neurologia medico-chirurgica. 1989 Dec:29(12):1113-8
[PubMed PMID: 2484190]
Level 3 (low-level) evidence
[23]
Assouad J, Riquet M, Foucault C, Hidden G, Delmas V. Renal lymphatic drainage and thoracic duct connections: implications for cancer spread. Lymphology. 2006 Mar:39(1):26-32
[PubMed PMID: 16724507]
[24]
Burnstock G, Loesch A. Sympathetic innervation of the kidney in health and disease: Emphasis on the role of purinergic cotransmission. Autonomic neuroscience : basic & clinical. 2017 May:204():4-16. doi: 10.1016/j.autneu.2016.05.007. Epub 2016 May 31
[PubMed PMID: 27270214]
[25]
Ferguson M, Ryan GB, Bell C. Localization of sympathetic and sensory neurons innervating the rat kidney. Journal of the autonomic nervous system. 1986 Aug:16(4):279-88
[PubMed PMID: 2427560]
[26]
Luff SE, Hengstberger SG, McLachlan EM, Anderson WP. Distribution of sympathetic neuroeffector junctions in the juxtaglomerular region of the rabbit kidney. Journal of the autonomic nervous system. 1992 Oct:40(3):239-53
[PubMed PMID: 1360993]
[27]
Tone K, Sakaguchi T, Anzai H, Yoshifuku K, Wada T. Epidemiological and bacteriological studies on an outbreak of Sh. sonnei. The Kitasato archives of experimental medicine. 1975 Mar:48(1):1-8
[PubMed PMID: 1104983]
Level 2 (mid-level) evidence
[28]
Johns EJ, Kopp UC, DiBona GF. Neural control of renal function. Comprehensive Physiology. 2011 Apr:1(2):731-67. doi: 10.1002/cphy.c100043. Epub
[PubMed PMID: 23737201]
[29]
Kopp UC, Cicha MZ, Smith LA. Endogenous angiotensin modulates PGE(2)-mediated release of substance P from renal mechanosensory nerve fibers. American journal of physiology. Regulatory, integrative and comparative physiology. 2002 Jan:282(1):R19-30
[PubMed PMID: 11742819]
Level 2 (mid-level) evidence
[30]
Kopp UC, Cicha MZ, Nakamura K, Nüsing RM, Smith LA, Hökfelt T. Activation of EP4 receptors contributes to prostaglandin E2-mediated stimulation of renal sensory nerves. American journal of physiology. Renal physiology. 2004 Dec:287(6):F1269-82
[PubMed PMID: 15292051]
[31]
Kopp UC, Cicha MZ, Smith LA, Mulder J, Hökfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of alpha1- and alpha2-adrenoceptors on renal sensory nerve fibers. American journal of physiology. Regulatory, integrative and comparative physiology. 2007 Oct:293(4):R1561-72
[PubMed PMID: 17699565]
Level 2 (mid-level) evidence
[32]
Liu L, Barajas L. The rat renal nerves during development. Anatomy and embryology. 1993 Oct:188(4):345-61
[PubMed PMID: 7506501]
[34]
Wang CS, Greenbaum LA. Nephrotic Syndrome. Pediatric clinics of North America. 2019 Feb:66(1):73-85. doi: 10.1016/j.pcl.2018.08.006. Epub
[PubMed PMID: 30454752]
[35]
Khanna R. Clinical presentation & management of glomerular diseases: hematuria, nephritic & nephrotic syndrome. Missouri medicine. 2011 Jan-Feb:108(1):33-6
[PubMed PMID: 21462608]
[36]
Chen A, Lee K, D'Agati VD, Wei C, Fu J, Guan TJ, He JC, Schlondorff D, Agudo J. Bowman's capsule provides a protective niche for podocytes from cytotoxic CD8+ T cells. The Journal of clinical investigation. 2018 Aug 1:128(8):3413-3424. doi: 10.1172/JCI97879. Epub 2018 Jul 9
[PubMed PMID: 29985168]
[37]
Luna-Antonio BI, Rodriguez-Muñoz R, Namorado-Tonix C, Vergara P, Segovia J, Reyes JL. Gas1 expression in parietal cells of Bowman's capsule in experimental diabetic nephropathy. Histochemistry and cell biology. 2017 Jul:148(1):33-47. doi: 10.1007/s00418-017-1550-z. Epub 2017 Mar 18
[PubMed PMID: 28315934]
[38]
Kuppe C, Leuchtle K, Wagner A, Kabgani N, Saritas T, Puelles VG, Smeets B, Hakroush S, van der Vlag J, Boor P, Schiffer M, Gröne HJ, Fogo A, Floege J, Moeller MJ. Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions. Kidney international. 2019 Jul:96(1):80-93. doi: 10.1016/j.kint.2019.01.037. Epub 2019 Feb 27
[PubMed PMID: 31029503]