Continuing Education Activity
Specular microscopy is an indispensable tool in ophthalmology, facilitating evaluation of the corneal endothelium, a pivotal aspect in eye health assessment and surgical planning. By offering detailed images showcasing the size, shape, density, and general state of endothelial cells, this non-invasive diagnostic method aids in the identification and management of various corneal conditions like Fuchs dystrophy and iridocorneal endothelial syndrome. Fluctuations in endothelial cell health serve as crucial indicators of disease presence and inform treatment decisions, underlining the significance of specular microscopy in clinical practice. Whether manual or automatic, the device's integration of computer-assisted analysis enables the quantification of essential endothelial parameters, facilitating accurate diagnoses, assessment of surgical risks, and patient prognosis elucidation.
This technology's capacity to capture high-resolution, magnified images empowers ophthalmologists to make informed decisions, particularly in surgical contexts, ultimately enhancing patient care and outcomes. Despite its limitations, including susceptibility to image quality variations and the need for expert interpretation, ongoing technological advancements promise to refine specular microscopy further, expanding its clinical applications and research potential. As the field progresses, specular microscopy remains available to guide ophthalmologists toward enhanced patient care and treatment strategies, reaffirming its status as a cornerstone in ophthalmic practice.
Objectives:
Determine the indications for specular microscopy.
Identify the contraindications of specular microscopy.
Evaluate the technique used during specular microscopy.
Identify the complications of specular microscopy.
Introduction
The corneal endothelium comprises a monolayer of hexagonal cells and it is imperative to assess the health of the corneal endothelium.[1][2] Specular microscopy is a noninvasive modality that can document the healthy and diseased endothelium photographically. Specular microscopy is also crucial in assessing the preoperative endothelial health before high-risk surgeries, comparing various techniques, the impact of lasers during refractive surgery, and the assessment of donor cornea before transplantation.[3] Specular microscopy represents a transformative advancement in ophthalmic imaging, providing an unparalleled window into the corneal endothelium, a critical layer of cells vital for maintaining corneal transparency and overall ocular health. This noninvasive photographic technique allows clinicians and researchers to observe and assess the endothelial cell layer at the back of the cornea directly, offering insights that were once inaccessible without invasive methods or post-mortem analysis.[4]
The various endothelial pathologies where specular microscopy plays an important role include Fuchs endothelial dystrophy, corneal dystrophies, posterior polymorphous dystrophy, pseudophakic bullous keratopathy, congenital hereditary endothelial dystrophy, viral endothelitis, ICE syndrome, trauma, uveitis and pharmacological disruption of the endothelium.[5]
Various specular microscopes are available for documenting endothelial cell details at various magnifications and calibrations.[4] Approximately 75 years ago, Vogt tried to obtain the endothelial cell morphology by using the reflected light of a slit lamp. However, limited magnification and rapid eye movements precluded a clear image. David Maurice first described specular microscopy in 1968.[6] In 1975, Laing first used the specular microscope for clinical use. A year later, Baurne et al used the specular microscope at 200X for rapid endothelial examination and photography. In corneal edema, the specular reflection is masked and hampers visualization of the corneal endothelium. Eye banks also need to assess the donor's corneal endothelial status.[7]
The endothelial cell layer is only one cell thick and is essential in preserving corneal dehydration and clarity. The cells act as a barrier to fluid from the aqueous humor and pump excess fluid from the stroma to prevent corneal swelling. Any dysfunction or decline in the cell count can lead to corneal edema and loss of visual acuity. Unlike other cells in the body, human corneal endothelial cells are post-mitotic: they do not regenerate. This places greater importance on monitoring their health and integrity to prevent and manage corneal diseases. The practice of specular microscopy in ophthalmology dates back to the early 20th century, but it was not until computer-assisted image analysis became available in the late 1970s and early 1980s that its use became more widespread.[2]
The innovation of non-contact specular microscopes allowed for a safer, more efficient assessment of the endothelium, making the process more comfortable for patients and convenient for practitioners. In a specular microscopy exam, light is directed toward the cornea and reflected off the inner endothelial layer. This reflection captures an image that can be analyzed for endothelial cell density (ECD), cell size (polymegathism), and shape (pleomorphism). ECD is a primary indicator of endothelial health, with a lower count suggesting a compromised cornea. Polymegathism and pleomorphism provide additional details regarding the uniformity and viability of the endothelial cells, with increased variation often indicating cellular stress or disease.[8]
The precision of specular microscopy has been instrumental in preoperative evaluations for intraocular surgeries, such as cataract extraction and corneal transplantation. The integrity of the endothelium is a critical determinant in patient selection and surgical prognosis, as iatrogenic trauma to this layer can have significant postoperative consequences. Consequently, the ability to accurately assess the endothelium has improved surgical outcomes and patient care. In corneal transplantation, specular microscopy is invaluable for determining the quality of donor corneas, ensuring that only tissues with a healthy endothelium are used for grafts.[9]
The technique has also revolutionized the management of corneal dystrophies and endothelial disorders such as Fuchs endothelial dystrophy and posterior polymorphous corneal dystrophy. In these conditions, specular microscopy can track disease progression and guide the timing of surgical interventions. In cases of acute or chronic corneal edema, the clarity provided by specular microscopy into the endothelial cell's health is paramount in formulating a treatment strategy.
Moreover, specular microscopy is not limited to disease management, as it plays a significant role in the fitting and managing of contact lenses, particularly in long-term wearers where endothelial damage is a concern.[10] It offers the means to monitor the long-term effects of contact lens wear on the endothelium, enabling early detection of adverse reactions and preventing potential complications.[11]
Specular microscopy provides quantitative and qualitative data that enhance research and clinical practice. In drug trials, the technique offers a metric for assessing drug toxicity in the cornea. Specular microscopy also aids in evaluating the impact of various ocular surgeries on the endothelium, thus influencing surgical techniques and postoperative care.[3]
However, specular microscopy is not without limitations. Image quality can be affected by factors such as corneal clarity, patient cooperation, and the presence of corneal pathology. In such cases, alternative methods like confocal microscopy may be necessary. Moreover, interpreting specular images requires significant expertise, as misinterpretation can lead to erroneous clinical decisions. Technological advancements continue to refine specular microscopy. Today, newer models boast increased automation, better image resolution, and user-friendly interfaces that streamline the process and enhance accuracy. Current research aims to develop software capable of more detailed analyses, potentially identifying endothelial changes before they manifest clinically.[4]
The continued evolution of specular microscopy will allow for its expanded use in clinical and research areas. With the burgeoning interest in regenerative medicine and cell-based therapies, the ability to monitor endothelial cell health is poised to become even more integral to ophthalmic practice. Specular microscopy thus remains a cornerstone in the field, not just as a tool for diagnosis and monitoring but as a beacon guiding ophthalmologists toward optimizing patient outcomes.[7]
Anatomy and Physiology
Anatomy of Corneal Endothelial Cells
Before discussing specular microscopy, it is important to understand the anatomy and physiology of corneal endothelial cells.[12] This is essential for the interpretation of specular microscopy results. The corneal endothelial layer is a monolayer of hexagonal cells. The hexagon shape allows for a more efficient and even distribution of membrane surface tension and gives the advantage of a larger surface area than its perimeter.[13]
These cells lie dormant in the G1 phase of the cell cycle, and there is no evidence of mitotic activity under normal conditions. When there is endothelial injury, the healing involves cellular elongation and spread to create a uniform cellular layer over the inner surface of the cornea.[3] This results in increased surface area and reduced endothelial cell count. When there is variation in cell areas of individual cells, it is called polymegathism or coefficient of variation. A perfect cornea should have 100% hexagonal cells. However, a normal cornea is expected to have 60% hexagonality. Under stress or insult to the endothelium, the cell count and hexagonality are reduced.[4]
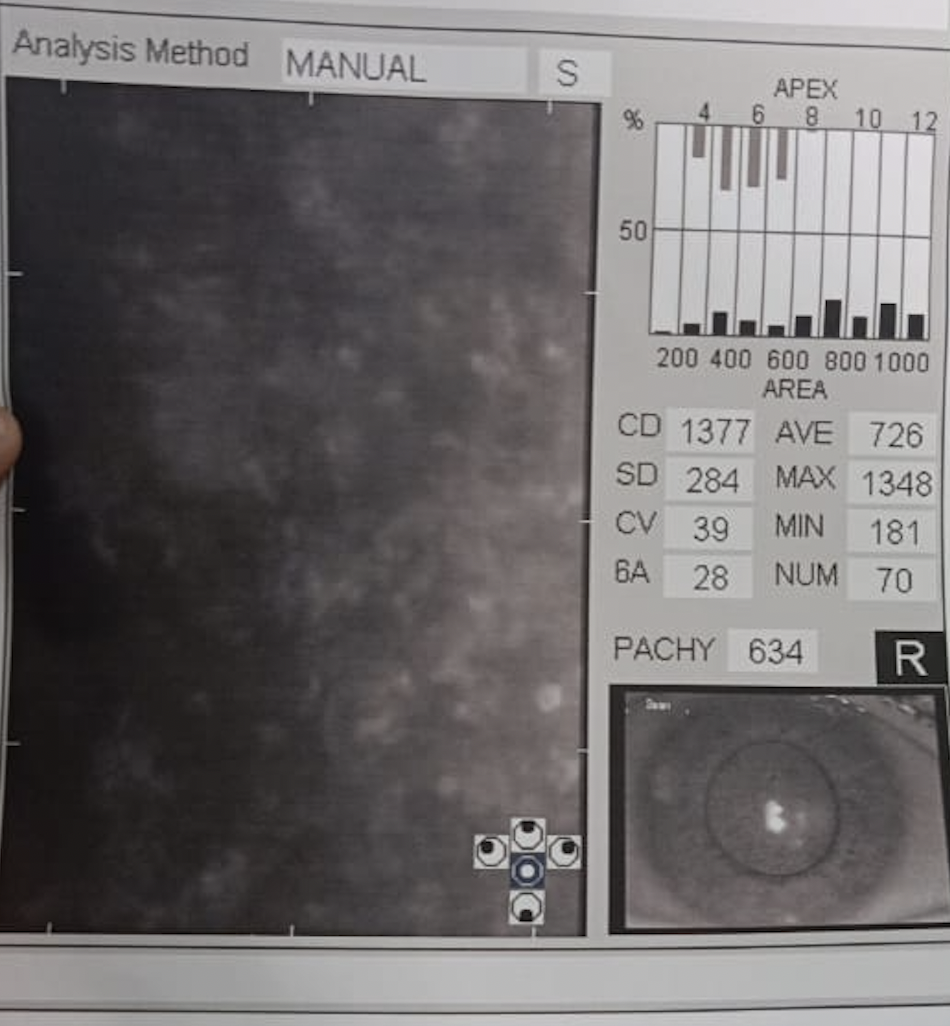
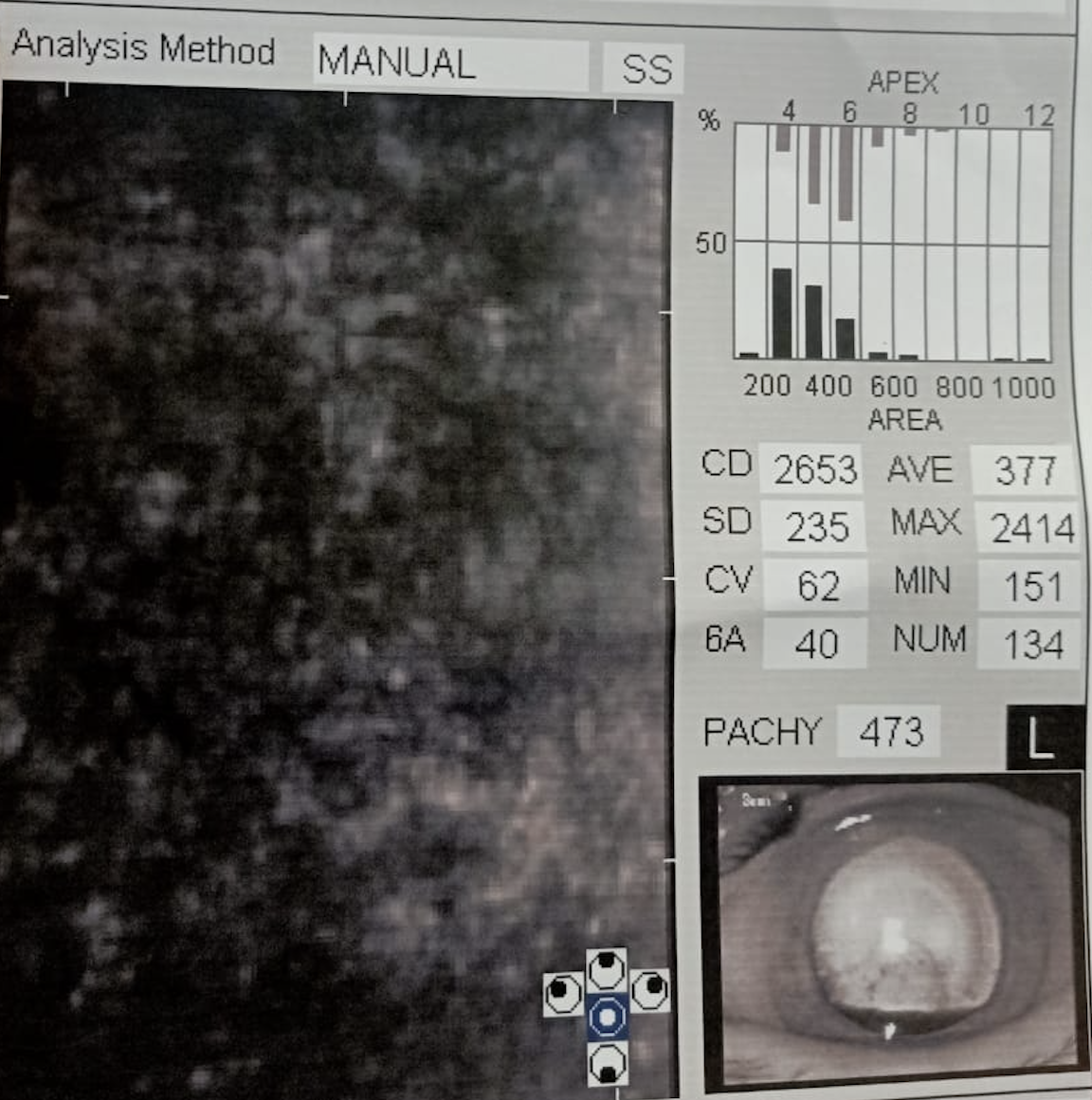
The endothelial cell's morphological analysis is characterized by polymegathism (coefficient of variation, CV), pleomorphism (% of hexagonal cells), cellular density (cells/mm), and cell area +/- SD (μm). The cell density is calculated by the formula 10/average cell area. Polymegathism is denoted by the coefficient of variation and is calculated by the formula CV= SD/mean cell area, where CV is the coefficient of variation and SD denotes the standard deviation of the cell area.[14]
The endothelial cell density reduces with aging at an average of 0.6% annually. This has 2 phases: rapid and slow.[15] The endothelial cell density is approximately 6000 cells/mm in the first month after birth and slowly declines to 3500 cells/mm by 5 years old, 3000 cells/mm at the age of 15 to 20 years, and 2500 cells/mm by the age of 50 years.[4]
This is a normal aging process in the cornea with a change in cellular dimensions and senescence of endothelial cells. The endothelial cell density also declines with racial, environmental, and geographical variations.[16] The endothelial cells lack regenerative capacity; any traumatic injury to the endothelial cells is repaired by the surviving adjacent cells. The most sensitive indices of corneal endothelial dysfunction are CV and its percentage of hexagonality for wound healing.[17]
The anatomy of the cornea is deeply intertwined with the utility and application of specular microscopy. The cornea's complex multi-layered structure and function must first be discussed to understand the role of specular microscopy. The cornea is the clear, dome-shaped surface that covers the front of the eye and plays a vital role in focusing vision. It is composed of 5 distinct layers, each with unique functions and properties:[1]
Epithelium: This is the outermost layer of the cornea and functions as a barrier against dirt, debris, and bacteria. It has regenerative capabilities and can repair itself from minor scratches or injuries.[18]
Bowman's layer: Located just beneath the epithelium, Bowman's layer is a tough sheet of tissue. It is formed from strong collagen fibers that protect the cornea's inner layers. Damage to this layer can lead to scarring, as it does not regenerate.[19]
Stroma: The stroma comprises most of the corneal thickness and consists mainly of water and collagen fibers. These fibers are organized precisely to maintain corneal clarity. Keratocytes (the cells within the stroma) are responsible for repairing and maintaining these collagen fibers.[20]
Descemet's membrane: This thin but tough sheet of tissue is the protective lining for the corneal endothelium. If injured, Descemet's membrane can regenerate, albeit slowly.[21]
Endothelium: The innermost corneal layer is a single layer of endothelial cells. These cells are critical for maintaining corneal dehydration and clarity. They pump excess water out of the stroma to prevent it from becoming swollen and opaque. Unlike the superficial epithelium, the endothelial cells have limited to no regenerative ability, so damage to this layer can have significant, long-lasting effects on vision.[17]
Specular microscopy specifically targets the endothelium. By using light reflection, it provides a clear image of this vital layer, allowing for the evaluation of cell density, shape, and size. The health of the endothelial cells, observed through this method, is a good indicator of the cornea's ability to maintain its necessary functions and transparency.[3]
Given the critical nature of the endothelium and its limited regenerative potential, specular microscopy has become an invaluable diagnostic tool in ophthalmology. It is used to diagnose and monitor corneal diseases, assess damage due to contact lens wear, evaluate candidates for refractive surgery, and ensure the viability of donor corneas for transplantation.
In summary, the relationship between corneal anatomy and specular microscopy is one of necessity and enhancement. Specular microscopy augments our understanding and ability to care for this complex and vital part of the eye, directly influencing the prevention, diagnosis, and treatment of corneal pathologies.
Physiology of Corneal Endothelial Cells
The monolayer of endothelial cells contains anywhere from 350,000 to 500,000 cells on the posterior surface of the cornea.[4] The major function of the endothelium is the formation of the Descemet membrane by secretion of the collagen matrix. The pumping activity of the endothelium helps maintain the cornea's health and transparency.[1]
The cornea is avascular and provides a physiological barrier to external stimuli.[22] The nutrient supply and diffusion of glucose and other solutes in the aqueous humor occur through the corneal endothelium. The aqueous influx into the stroma from the anterior chamber is due to a change in intraocular pressure, which facilitates diffusion.[23]
The endothelium plays a significant role in maintaining hydration of the cornea to prevent edema. The endothelium acts as a physiological and anatomical barrier to maintain corneal hydration, which makes it semipermeable.[17] On the lateral aspect of the cell membrane, tight junctions between the endothelial cells help control the endothelial permeability and the amount of fluid influx in the corneal stroma.[24]
The endothelium also has an active Na+/K+ ATPase pump and carbonic anhydrase pump that maintains stromal deturgescence by fluid efflux from the stroma by a dynamic transport mechanism. The carbonic anhydrase pump also helps transport bicarbonate ions across the cell membrane, allowing the aqueous flow in the anterior chamber.[23]
The physiology of corneal endothelial cells is a critical aspect of ocular health, and understanding it is essential for recognizing the significance of specular microscopy in ophthalmology. Corneal endothelial cells form a single layer of hexagonally shaped cells on the innermost surface of the cornea, known as the endothelium. Despite being only one cell thick, this layer regulates corneal hydration, which is crucial for maintaining corneal transparency and, as a result, clear vision. The physiology of these cells can be broken down into several key functions:[17]
Barrier Function: Corneal endothelial cells serve as a barrier to prevent the influx of excess fluid from the aqueous humor into the corneal stroma. This function is critical to preserving the cornea's deturgescence (its state of relative dehydration), which is vital for its transparency.[17]
Pump Function: Endothelial cells actively pump ions, particularly bicarbonate and chloride ions, from the stroma into the aqueous humor. Water follows these ions osmotically, which helps to keep the cornea in its deturgescent state. This ionic transport is driven by enzymes like Na+/K+ ATPase on the cell membranes.[25]
Cell Density and Replication: Corneal endothelial cells are post-mitotic in humans, which means they do not replicate after a person reaches adulthood. As a result, cell density decreases with age or trauma, as cells spread out to cover voids left by lost or damaged cells. The remaining cells enlarge (polymegathism) and take on varied shapes (pleomorphism) to maintain continuous coverage. Healthy corneal endothelium has a high cell density with a uniform hexagonal shape. Specular microscopy is instrumental in evaluating these aspects.[26]
Response to Injury: When endothelial cells are lost due to injury or disease, neighboring cells enlarge and migrate to cover the defect. However, they do not divide to replace lost cells. Over time, significant loss of endothelial cells can compromise the endothelium's ability to function properly, potentially leading to corneal edema and loss of vision.[17]
Metabolic Activity: Corneal endothelial cells are metabolically active, generating the ATP necessary for the pump function. They predominantly utilize aerobic metabolism but can switch to anaerobic glycolysis when necessary.
Understanding the physiology of these cells is vital for ophthalmic diagnoses and treatments involving the cornea. Specular microscopy allows clinicians to assess the health and function of the endothelium by providing detailed images that can be analyzed for cell density, size, and shape. It helps detect early signs of endothelial dysfunction, guides the management of corneal diseases, and informs decisions related to surgeries that may affect the cornea, like cataract removal or corneal transplants.
In summary, the corneal endothelial cells play a central role in maintaining corneal clarity through their barrier and pump functions. The health of these cells, as visualized by specular microscopy, is paramount for ophthalmologists in ensuring the integrity of the cornea and in making critical clinical decisions.[3]
Indications
Specular microscopy gives a peculiar and distinctive endothelial picture in various corneal endotheliopathies, as discussed below. The corneal endotheliopathies are divided into primary and secondary. Primary endotheliopathies are not secondary to any ocular or systemic pathology. In secondary endotheliopathy, the endothelial damage is secondary to an underlying ocular or systemic insult.[2]
Examples of Primary Endotheliopathies
- Corneal guttata
- Fuchs endothelial corneal dystrophy
- Posterior polymorphous endothelial dystrophy
- Congenital hereditary endothelial dystrophy
- Iridocorneal endothelial syndrome
- Senile endothelial degeneration [2]
Examples of Primary Endotheliopathies
- Endothelial changes after surgery
- Contact lens endotheliopathy
- Pseudoexfoliation induced endotheliopathy
- Endotheliopathy after ocular inflammation (eg, uveitis, endothelitis)
- Endotheliopathy after forceps injury
- Endotheliopathy in corneal dystrophy
- Drug-induced endotheliopathy [2]
Corneal Guttata
The most common primary corneal endotheliopathy is corneal guttata. They are seen as a part of the normal aging process.[17] These are also evident in some pathologies like Fuchs endothelial dystrophies, secondary to inflammation and trauma. Guttae are present in the central cornea and have minimal to no effect on visual acuity. Up to 70% of the population older than age 40 can have corneal guttae.[17] They are best visualized by the specular reflection technique and are observed as dark areas resembling a hole in the endothelial mosaic. The dark areas form because the apex of the gutta lies outside the specular plane of focus. The guttae have 5 stages of development which are observed on specular microscopy.[3]
- Stage 1: Nodular, small, dark structure in the endothelial cell center
- Stage 2: Nodules are of the same size as endothelial cells; the surrounding cells are stretched
- Stage 3: Very large, multiple guttae may be seen, and one nodule has many endothelial cells; the adjacent cells lose their boundaries
- Stage 4: The guttae coalesce, and endothelial cells in between appear abnormal
- Stage 5: There is a continued coalescence of guttae, and the endothelial mosaic becomes challenging to visualize
Fuchs Endothelial Corneal Dystrophy
FECD is characterized by the presence of guttae, which are focal excrescences of DM in the central cornea.[27] The guttae may or may not affect visual acuity, and there is no direct correlation between guttae and loss of visual acuity. Guttae morphology can vary from fine, coarse, and confluent to nonconfluent, and their number can vary from few to numerous.[28]
These appear as drop-out areas on the endothelial image (see Image. Fuchs Endothelial Corneal Dystrophy). In the specular images with few discrete and nonconfluent guttae, the endothelial cells between the dropout areas can still be made to make a precise diagnosis. However, when there are numerous guttae, the endothelial cells cannot be imaged, and the specular images are not readable to make a correct diagnosis.[4] The specular image should always be correlated clinically, and the limitations of the imaging in FECD should be well understood.
The functionality of endothelium should be associated primarily with the patient's signs and symptoms, such as early morning blurring of vision, subepithelial haze, and central versus peripheral pachymetry changes. The changes in the FECD endothelium start from the center and progress towards the periphery.[6]
One method of assessment of endothelium is to perform descematorhexis without endothelial keratoplasty. Specular microscopy should be obtained in different gazes to have a preoperative workup of the patient and help in decision-making.[3] The peripheral corneal endothelial cell's health will help assess patients who are considered for DWEK. The peripheral endothelial count should be above 1000 cells/mm. When FECD and cataract coexist, the decision for endothelial keratoplasty is based on patient symptoms, pachymetry changes, and specular count.[29]
Good specular count with minimal guttae can be managed with cataract surgery with a good viscoelastic cover of the endothelium. While subjecting the patient to cataract surgery, guarded prognosis and chances of corneal decompensation should be explained to the patient.[4]
Sometimes, the guttae can be misleading and mimic many conditions, such as pigments in anterior uveitis, pseudoguttae, or secondary guttae as in ocular inflammation, infection, endothelial swelling, or Hassal-Henle bodies, which are DM excrescences in the peripheral cornea. Pseudoguttae are transient and reverse when the pathology reverses. Hence the specular finding should always be correlated with slit lamp imaging.[30]
Posterior Polymorphous Endothelial Dystrophy
The presence of vesicles, band-shaped structures, and placoid mosaics on the endothelium characterizes this condition. The pathology can be bilateral or unilateral. In many patients, the endothelial changes are subtle and can be easily overlooked.[2]
Posterior polymorphous endothelial dystrophy can be mistaken for keratoconus due to steep cornea, and unilateral cases may result in amblyopia. Specular microscopy picks up these cases. The endothelial cell density is reduced in these cases compared to age-matched eyes, and endothelial cells are missing in the areas of vesicles and bands.[31]
Congenital Hereditary Endothelial Dystrophy
Stromal haze in CHED is a barrier in specular imaging and precludes a clear image of the endothelium. Hence, it is not possible to obtain a clear image in these cases.[31]
Iridocorneal Endothelial Syndrome
ICE syndrome comprises Chandler syndrome, progressive iris atrophy, and Cogan-Reese syndrome. Specular microscopy gives distinct morphological changes in these cases.[32] There are 2 grading systems for describing the endothelial changes: Hirst's and Sherrard's. In these cases, specular microscopy reveals rounded cell borders, increased areas of blackouts, and a dark and light reversal pattern of normal endothelium.[4]
Senile Endothelial Degeneration
With advanced age, there is an abnormal reduction in endothelial cell density and an increase in pleomorphism, polymegathism, and proliferation in guttae.[17]
Endothelial Changes after Surgery
A variable degree of endothelial cell loss has been reported after intraocular surgeries such as cataract surgery and phakic IOLs.[33] As a result, specular count reveals a lower count and larger mean cell area in the pseudophakic eye versus the age-matched group. There is also a good amount of endothelial cell loss after keratoplasty, and it appears rapidly after the initial years of penetrating keratoplasty.[34]
Approximately 4% to 10% of endothelial cell loss occurs after routine cataract surgery. This postoperative cell loss is called iatrogenic endotheliopathy. This results in corneal decompensation and stromal edema. The various risk factors for iatrogenic endotheliopathy include ocular surgery, diabetes mellitus, uncontrolled glaucoma, uveitis, polymegathism, pleomorphism, and corneal guttae in FECD.[2]
One study by Armitage et al demonstrated biexponential cell loss after keratoplasty. In another study, endothelial cell loss was 7.8% per year between 3 and 5 years post-keratoplasty and 4.2 per year between 5 and 10 years. The mean 5-year endothelial cell loss after endothelial keratoplasty has been reported to be 48%.[35]
Contact Lens Endotheliopathy
Regular contact lens wear has been reported to cause acute and chronic corneal endothelium changes as contact lens wear duration increases and the rate of polymegathism increases.[36] Wearing contact lenses for a longer duration reduces oxygen permeability, which results in hypoxia. Chronic hypoxia causes stromal edema and later decompensation. Discontinuing contact lenses doesn't result in acute reversal of endothelial morphological changes.[37]
However, the changes reverse over an extended period or if an alternate contact lens is used with higher oxygen permeability. Contact lens-induced endotheliopathy results in corneal edema, foreign body sensation, photophobia, reduced visual acuity, and corneal exhaustion syndrome.[38]
Pseudoexfoliation Induced Endotheliopathy
Pseudoexfoliation is known to cause endothelial cell alteration. This results in reduced cell density and guttae.[39]
Endotheliopathy after Glaucoma
Uncontrolled IOP may reduce endothelial cell density and morphological changes in the endothelium. Another school of thought is physiological alternation in the glaucomatous eyes, reduced aqueous outflow, or decreased oxygen concentration of the aqueous.[40]
Endotheliopathy after Ocular Inflammation (Uveitis, Endothelitis)
Uveitis can result in reduced endothelial cell loss and cell function. In uveitis, immune-reactive proteins are released in the anterior chamber, leading to endothelial cell loss.[41] The inflammatory cell can penetrate the junction between endothelial cells during acute inflammation. This results in cells dislodging and free-floating in the aqueous. In acute anterior uveitis, specular microscopy reveals dark dropout areas located at the endothelial cell intersections, invading the large white blood cells.[2]
Endotheliopathy after Forceps Injury
Forceps trauma can lead to DM rupture and result in corneal edema at birth. The tear heals, and corneal edema is resolved with the migration of endothelial cells to cover the defect. The endothelial cell density is lower in the eyes compared to the normal.[3]
Endotheliopathy in Corneal Dystrophy
In macular corneal dystrophy (MCD), there is the deposition of GAGs in the stroma and corneal endothelium. In MCD, guttae similar to FECD are observed. Endothelial morphological changes and reduced cell density can occur in these cases after cataract surgery. A proper preoperative workup is necessary.[42]
Drug-Induced Endotheliopathy
Patients who are on medication for diabetes and hemodialysis for chronic kidney disease can have endothelial abnormalities.[43] These patients undergoing cataract surgery should be thoroughly evaluated to avoid the risk of corneal decompensation. Certain medications can lead to endothelial cell loss and result in bilateral corneal edema. Antiparkinson medications, toxins like madar juice (calotropis), and plant-based medications can result in drug-induced epitheliopathy.[44]
Role of Specular Microscopy in Eye Banks
Assessing the donor corneal endothelial status before subjecting the tissue to keratoplasty is a regular and standard practice.[34] The microscopes available in the eye banks help magnify views of endothelial cells. An ex-vivo wide-field specular microscope with dual imaging characteristics is employed to assess a larger area of the endothelial surface. The donor tissue is warmed to 25 °C before the examination, ranging from 1 to 2 hours to obtain a clear endothelial image.
The endothelial count is an imperative assessment parameter to decide donor suitability for optical penetrating or lamellar keratoplasty. The cut-off point for OPK is 2000 cells/mm; for lamellar transplant, it is 2200 to 2500 cells/mm. These values vary based on the eye bank and surgeon preference.[4] A donor count of less than 2000 is employed for either therapeutic, tectonic, or research and education purposes.[45]
Contraindications
The contraindications of specular microscopy include:
- Uncooperative patient
- Corneal edema
- Highly irregular endothelium
- Patient unable to fixate
- Nystagmus
- Severe ptosis [8]
Specular microscopy is a pivotal diagnostic tool used in ophthalmology to examine the corneal endothelium, the innermost layer of the cornea consisting of a single layer of cells. This non-invasive technique provides critical information on cell density, morphology, and the overall health of the endothelium, aiding in the diagnosis of endothelial disorders, pre-surgical evaluation, and postoperative monitoring. Despite its significant benefits, certain conditions and situations are contraindications to performing specular microscopy due to the potential harm to the patient or the likelihood of obtaining inaccurate or uninterpretable results.[46]
Absolute Contraindications
Severe corneal opacification
Severe opacities in the cornea, whether due to edema, scarring, or dystrophic changes, can prevent the specular microscope from capturing clear images of the endothelial layer. In these cases, the light necessary for imaging cannot penetrate the cornea effectively, rendering the examination impractical.[3]
Active ocular Iinfections
Performing specular microscopy in active ocular infections, especially with contact specular microscopes, can risk exacerbating the infection or spreading it to other parts of the eye. This is particularly concerning in cases of viral, bacterial, or fungal keratitis.[47]
Acute ocular trauma
In the setting of recent ocular trauma, introducing any instrumentation, even non-contact types, may pose a risk of further injury or infection. Specular microscopy in such situations is best postponed until the eye has adequately healed.[48]
Relative Contraindications
Shallow anterior chamber
A shallow anterior chamber poses a challenge for contact specular microscopy, as the risk of corneal touch and potential injury increases. Non-contact specular microscopy might be safer, though patient cooperation and positioning become even more critical.[49]
Severe photophobia
Patients with sensitivity to light may find specular microscopy, which requires bright illumination for endothelial imaging, uncomfortable or intolerable. In such instances, patient distress might preclude successful examination.[50]
Pediatric Patients and Uncooperative Patients
Specular microscopy requires the subject to remain still with open eyes for several seconds. Infants, young children, or patients who cannot cooperate due to cognitive or psychiatric reasons may not be suitable candidates for this examination.[51]
Advanced corneal dystrophies
In advanced stages of corneal dystrophies that involve the endothelium, such as Fuchs endothelial corneal dystrophy, the altered endothelial morphology might make it difficult to obtain meaningful measurements or images, especially if significant corneal edema is present.[10]
Considerations and Alternatives
In cases where specular microscopy is contraindicated or not feasible, alternative diagnostic techniques should be considered. Optical coherence tomography (OCT), particularly anterior segment OCT, can provide valuable information about the cornea and its layers without direct contact or illumination. Ultrasound pachymetry can offer corneal thickness measurements, indirectly indicating endothelial health, especially in edematous conditions. Clinicians must evaluate each patient's condition, the risks and benefits of specular microscopy, and the availability of alternative diagnostic methods before proceeding. Patient safety, comfort, and the quality of diagnostic data should guide the decision-making process.[52]
Equipment
Contact Specular Microscope
In this microscope, a contact lens with a coupling fluid is used. This has a similar index of refraction to that cornea, which eliminates the corneal specular reflection. In this method, the corneal thickness also includes the contact lens thickness.[8]
The reflection from the contact lens surface replaces that of the corneal surface. The contact apparatus provides good resolution as well as magnification. Due to the contact procedure, there is a risk of infection if sterile precautions are not taken, and the patient often feels uncomfortable. Manipulation with this technique can induce artifacts, especially in diseased corneas.[53]
Noncontact Specular Microscope
In a non-contact specular microscope, the reflections from the anterior surface are eliminated by increasing the angle of incidence. By modifying the angle of incidence, the anterior review is moved to the side, and there is less specular reflection. This non-contact technique is more tolerated and accepted by the patient, and there is a minor infection. Hence, a broader view is obtained.[8]
Wide Field Specular Microscope
This is a modification of the previous version with the addition of a scanning mirror. A field of 800 μm is obtained with no loss of contrast.[54] This technique allows a continuous view of an 800-μm diameter area because of the mirror's higher rate of oscillation. The wide field provides for a 10 to 15 times larger picture, with higher resolution and image quality is less affected by eye movements. A wide field provides a more accurate cell count, the topography is easily evaluated, and the relocation of a particular portion of endothelium is easy.[55]
Personnel
Paramedical technicians having working knowledge and being well-trained in handling specular microscopy is necessary for effective patient management. The optometrists help in assessing the visual as well as the refractive status of the patient. The ophthalmic corneal surgeon, well-trained in evaluating specular count, helps delineate the endothelial pathology and provides the targeted treatment. The mid-level ophthalmic personnel also assist in patient counseling and follow-up.[6]
Preparation
Preparing for specular microscopy involves several steps to ensure patient comfort and to obtain accurate results from the examination. Here's an outline for preparation:
Pre-Examination Preparation
Patient education: The procedure is explained to the patient to ensure they understand the purpose and what to expect during the examination. Clear communication can help alleviate any anxiety and promote cooperation.[56]
Medical history review: The patient’s medical and ocular history is reviewed, paying close attention to any conditions that may contraindicate the use of specular microscopy.[57]
Contraindication assessment: Absolute contraindications, including active eye infections or acute corneal trauma, are ruled out. Relative contraindications must be evaluated and discussed with the patient.[48]
Consent: Informed consent is obtained after discussing the risks, benefits, and any alternatives to the procedure.[58]
Day of the Examination
Patient arrival: On the examination day, consent is reaffirmed, and any last-minute questions the patient may have are answered.
Topical anesthetic (if necessary): For contact specular microscopy, a topical anesthetic is instilled to numb the cornea to ensure patient comfort. Non-contact methods typically do not require anesthetic.
Positioning the patient: The patient is seated comfortably at the specular microscope with their chin in the chin rest and forehead against the support. Proper alignment is crucial for optimal imaging.[59]
Fixation target: If available, the patient's eye is directed towards a fixation target to help keep the eye still and properly aligned during the imaging process.
During the Examination
Image acquisition: Images of the corneal endothelium are acquired. The patient must remain still during this process to ensure the clarity of images. Multiple images may be needed for accuracy.[60]
Patient instructions: During the process, clear instructions are given to the patient. For example, they can be asked to blink normally and keep their eyes open during image capture.
Post-Examination
Patient comfort: The patient should be asked about their comfort, especially if a contact method is used, as the eye might feel irritated once the anesthetic wears off.[61]
Instruction for aftercare: If anesthetic drops were used, the patient should not rub the eye until full sensation returns. No specific aftercare is needed for non-contact methods.
Data analysis: After the patient has left, the images captured are analyzed to assess the endothelial cell count, cell size variation, and presence of any abnormal cells or patterns.
By following these preparation steps, clinicians can maximize the likelihood of a smooth procedure and accurate assessment of the corneal endothelium.[62]
Technique or Treatment
The procedure is first fully explained to the patient to relieve any anxiety and make the patient comfortable. The cornea is systemically scanned, and all quadrants should be scanned centrally, superior, inferior, temporal, and nasal. The specular microscope is a light-reflecting microscope.[1]
The slit beam of light is projected onto the cornea, and the light reflected from the tissue interface helps form the image. The difference between the refractive index of aqueous humor and endothelial cells gives rise to specular or mirror-like reflection on the posterior surface.[4]
The reflected light is approximately 0.02% of the incident beam of light. If a broad beam of light is used, the reflected beam from the stroma and epithelium obscures a clear view of the endothelium. Liang described 4 distinct zones on specular microscopy using a slit beam of evaluation:[63]
- Zone 1: Epithelium/ Coupling fluid of lens
- Zone 2: Corneal stroma
- Zone 3: Endothelium
- Zone 4: Aqueous humor
Dark boundary: The area between zone 3 and 4 is dark and is called a dark boundary.[3]
Light boundary: The area between zone 3 and zone 2 is dark and is called a light boundary.[3]
There are various ways to get quantitative information on the endothelium, such as the corner, comparison, frame, center to center, and flex center. Errors can also occur while evaluating the corneal endothelium.
Frame method: In the frame method, cell density can be assessed by counting the number of cells within a frame.[4]
Comparison method: In the comparison method, cell density is assessed by visually comparing the image of a known set with a hexagonal pattern of various cell densities.[4]
Corner method: In the corner method, the intersecting sides of the endothelial image frame are located.[4]
Fixed frame method: This method can induce many errors when many border cells are present. This error can be eliminated by the variable frame counting method and is preferable to the fixed-frame method.[3]
Tracing analysis: This refers to tracing the individual cell areas and other parameters.[3]
Digested cell analysis: The cells can be digested after tracing outlines using a digested tablet. The analysis can be done using a photograph, a negative image on a television screen, or a videotaped recording.[3]
Computerized analysis: The computerized analysis helps to assess the cell density, frequency, size, and shape.[3] Subjective decision-making can induce errors in the center-to-center method and cell border. The accuracy of endothelial cell morphology depends on the clarity and quality of the endothelial scan obtained. The specular scans are based on the specular reflex. Hence, any barrier in the endothelial monolayer's optical pathway will affect the scan quality. The other factors hampering scan quality include tear film abnormalities, epithelial haze, stromal scar, guttae, and DM damage. Based on the quality of the endothelial scan, it can be graded as good, fair, poor, or impossible.[64]
Automated specular microscopy: In this method, automatic analysis of individual cell layers is done, which are well delineated. If the cell boundaries are not visible, then the manual method should be used to perform a specular count. The interobserver subjective variability can be up to 6% for the excellent quality of endothelial images and 6% to 11% for acceptable quality.[3]
Complications
Specular microscopy is a safe and non-invasive procedure for evaluating the cornea's endothelial layer. However, like all clinical procedures, it has the potential for complications, though these are rare considering the non-contact nature of most specular microscopes today. Possible complications that could arise from specular microscopy include:[4]
For Contact Specular Microscopy
Corneal abrasion: If the probe touches the cornea inadvertently, the most common complication would be a corneal abrasion. This can cause pain, a foreign body sensation, and an increased risk of infection.[65]
Infection: Although extremely rare, any contact with the ocular surface can introduce pathogens, leading to infections like keratitis, especially if proper sterilization protocols are not followed.[66]
For Noncontact Specular Microscopy
Discomfort from bright light: The intense light needed to illuminate the cornea for imaging can cause temporary discomfort or photophobia, especially in patients with light sensitivity.[3]
Eye fatigue: Patients may experience eye fatigue or a headache from the need to fixate on a target for a prolonged period during image capture.[67]
General Complications
Allergic reaction: If a topical anesthetic is used in contact specular microscopy, there is a potential for an allergic reaction to the medication.
Tearing or blinking reflex: The bright light and the instrument's proximity can induce tearing or a blinking reflex, which could hinder the image acquisition process.[68]
Psychological distress: Some patients may experience anxiety or psychological distress due to the proximity of the instrument to the eye, especially in a contact procedure.[69]
For Patients with Preexisting Conditions
Patients with preexisting ocular conditions (eg, a shallow anterior chamber) or those who are postoperative may be at an increased risk for complications when undergoing contact specular microscopy. With modern non-contact specular microscopy, the risk of complications is minimal. When complications do occur, they are usually minor and can be managed effectively with appropriate clinical care. The procedure should always be performed by trained personnel to minimize these risks, and proper patient selection and preparation can further reduce the chance of complications.[70]
Clinical Significance
Specular microscopy is challenging to ascertain in patients with small micromovements and nystagmus.[5] It gives information only about the morphological status and not the functional level of the endothelium. However, it is a vital tool for assessing the endothelial status in primary and secondary corneal endotheliopathies. Specular microscopy has revolutionized the management of FECD and patients with cataracts and is also an important tool for assessing the endothelial status on serial follow-ups. Understanding and working knowledge of specular microscopy are imperative for ophthalmologists to guide the management and prognosis of all patients.[3]
Specular microscopy is a clinical imaging technique that is essential for the evaluation of corneal endothelial health. This non-invasive method allows for detailed assessment of the endothelial cell layer, providing clinicians with quantitative and qualitative data critical for diagnosing endothelial pathologies, managing corneal diseases, and making surgical decisions. The clinical significance of specular microscopy can be discussed under several key aspects:[6]
Evaluation of Endothelial Cell Density
Endothelial cell density (ECD) is a primary measure of corneal health. A healthy adult cornea typically has an ECD of 2500 to 3000 cells/mm2. Specular microscopy allows for directly counting these cells in a given area, providing an accurate measure of ECD. A low ECD can indicate endothelial dysfunction or be a sign of corneal pathologies, such as Fuchs dystrophy or endothelitis.[71]
Assessment of Endothelial Cell Morphology
Beyond cell density, specular microscopy gives insight into cell shape and size, collectively known as cell morphology. Healthy endothelial cells are typically uniform in size and hexagonal in shape. Polymegathism (variation in cell size) and pleomorphism (variation in cell shape) can indicate cell stress or corneal endothelial dystrophy.[72]
Preoperative Evaluation
Specular microscopy is critical before cataract surgery or corneal transplantation to assess whether the endothelium can withstand the additional stress of surgery. The procedure helps predict the potential for corneal decompensation postoperatively, guiding both the surgical technique and the postoperative management.[73]
Postoperative Monitoring
Specular microscopy is used after corneal surgery to monitor the health of the endothelium. This technique is particularly important in the follow-up of patients with corneal transplants, as it can detect early graft failure or rejection. In cataract surgery patients, it helps monitor for potential endothelial damage caused by surgical trauma or intraocular lens placement.[74]
Screening for Endothelial Dystrophies
Specular microscopy is the gold standard for diagnosing endothelial dystrophies like Fuchs' endothelial corneal dystrophy. It allows for early detection by identifying characteristic guttae (droplet-like accumulations of Descemet's membrane) and changes in cell density and morphology before significant vision loss occurs (see Image. Guttata).[3]
Contact Lens Fitting
For patients who wear contact lenses, particularly rigid gas-permeable lenses, specular microscopy can assess the endothelium for any signs of hypoxia or mechanical trauma, ensuring the continued safe use of contact lenses.[75]
Drug Toxicity and Corneal Edema Assessment
Certain medications, such as topical glaucoma therapies, can have toxic effects on the corneal endothelium. Specular microscopy helps assess patients for signs of drug toxicity and evaluates corneal edema to determine whether it is due to endothelial dysfunction.[2]
Research and Epidemiological Studies
Specular microscopy plays a significant role in research settings for understanding the pathophysiology of corneal diseases and for epidemiological studies assessing the health of populations' corneal endothelium, providing invaluable data for advancing ophthalmic knowledge.[76]
Conclusion
Given its ability to provide a detailed view of the corneal endothelium, specular microscopy's clinical significance cannot be understated. This non-invasive tool has become indispensable in modern ophthalmology, guiding the management of various corneal conditions, enhancing surgical outcomes, and contributing to the broader understanding of ocular health. As technology advances, specular microscopy offers improved imaging and analysis techniques, further expanding its role in clinical care and research.[4]
Enhancing Healthcare Team Outcomes
It is essential for ophthalmologists, optometrists, specular trained technicians, and nursing staff to work in tandem to better manage patients with endothelial pathologies. These patients can gain good visual acuity if managed well.[77]
In specular microscopy, enhancing healthcare team outcomes involves ensuring that the personnel involved in this diagnostic procedure are well-trained, the equipment is used properly, and the results are accurately interpreted to facilitate the best possible patient care. Effective strategies include:[78]
Interdisciplinary training: Ensuring that all team members, including ophthalmologists, optometrists, technicians, and nurses, are trained in the use and interpretation of specular microscopy images. Standardizing training can improve consistency in outcomes.[79]
Standard operating procedures (SOPs): Developing and following SOPs for operating specular microscopes and patient preparation can reduce variability in test results.[80]
Quality control measures: Implementing quality control measures, such as regular equipment calibration and maintenance, can ensure that the specular microscope provides accurate readings.[4]
Continuing education: Staying updated with the latest research and advancements in specular microscopy can enhance the team's knowledge and skill set, leading to better patient outcomes.[81]
Team communication: Fostering open communication among healthcare team members can lead to more effective problem-solving and sharing of best practices.
Patient education: Equipping the team to educate patients about the importance of specular microscopy in diagnosing corneal diseases can improve patient compliance and follow-up.[82]
Data utilization: Efficiently utilizing the data from specular microscopy to make informed decisions regarding patient treatment plans, surgical interventions, and post-operative care.[83]
Outcome monitoring: Regularly reviewing and analyzing patient outcomes to identify areas for improvement in the specular microscopy process.
By focusing on these areas, healthcare teams can enhance their effectiveness and the quality of care they provide, specifically when utilizing specular microscopy as part of their diagnostic toolkit.[82]
Nursing, Allied Health, and Interprofessional Team Interventions
The nursing and allied health staff working as an interprofessional team help perform the specular scan and guide the patients regarding the need for further intervention and follow-up.[84] "Nursing, Allied Health, and Interprofessional Team Interventions in Specular Microscopy" focuses on the collaborative roles and actions taken by various healthcare professionals to optimize the use and outcomes of specular microscopy. Discussion on the topic is as follows:
Nursing Interventions
Patient Education and Preparation
Nurses play a key role in educating patients about the purpose and process of specular microscopy, helping them understand what to expect during the procedure. They can also instruct patients on potentially removing contact lenses before the procedure and discuss any contraindications or precautions.[85]
Patient Support and Comfort
Ensure the patient is comfortable and positioned correctly for the procedure. Manage any anxiety or discomfort associated with the procedure, providing reassurance as needed.[86]
Allied Health Contributions
Ophthalmic Technicians
Technicians are often responsible for the actual operation of the specular microscope. They ensure that the equipment is properly calibrated and functioning. They can also take on some patient education roles, explaining the technical aspects of the procedure.[87]
Optometrists
In settings where optometrists are involved, they may perform specular microscopy and interpret the results as part of routine eye examinations or in specialty contact lens fittings.[88]
Interprofessional Team Coordination
Collaboration for Diagnosis and Treatment
Interprofessional teams, including ophthalmologists, optometrists, nurses, and technicians, should collaborate to interpret the results of specular microscopy and integrate them into the patient's overall care plan. Regular team meetings can be helpful to discuss complex cases and share insights from specular microscopy results.[89]
Quality Improvement
The interprofessional team should engage in continuous quality improvement initiatives to enhance the accuracy of readings and patient outcomes. They may review cases retrospectively to identify any discrepancies in interpretation or opportunities for protocol refinements.[90]
Research and Development
Contributions from various team members can be valuable in conducting research related to corneal diseases and the role of specular microscopy in their management. Teams can also contribute to developing new techniques or improvements in specular microscopy.[78]
Case Management
In cases where specular microscopy reveals significant corneal pathology, case managers or other team members may coordinate additional care, such as referrals to corneal specialists or arranging for corneal transplantation.[3]
Ensuring Access and Follow-up
Teams may work to ensure patients can undergo specular microscopy when needed, including managing appointments and follow-up care. Nursing and allied health professionals can track patient compliance with treatment plans informed by specular microscopy findings. In conclusion, specular microscopy is a tool that benefits greatly from interprofessional collaboration. The combined expertise of nursing, allied health professionals, and the broader healthcare team is critical in optimizing the utilization of specular microscopy for the diagnosis and management of corneal conditions. Each discipline brings unique skills and perspectives, which can enhance patient care and outcomes when effectively integrated.[91]
Nursing, Allied Health, and Interprofessional Team Monitoring
The nursing, allied health staff, and interprofessional team help assess and evaluate specular microscopy of these patients with regular follow-up. The skilled staff can understand the progression or stability of endotheliopathy by assessing the specular scans and also help in counseling these patients.[84] The phrase "Nursing, Allied Health, and Interprofessional Team Monitoring in Specular Microscopy" suggests a collaborative approach to monitoring patient care and outcomes within the context of specular microscopy. Here's how these roles may interplay:
Nursing Role
Monitoring Patient Response
Nurses are pivotal in monitoring patients before, during, and after the procedure, looking for any adverse reactions or complications related to specular microscopy.[92]
Documentation
Accurate documentation by nursing staff of the patient's condition and response to the procedure is vital. This includes noting any changes in the corneal appearance or patient-reported symptoms.[93]
Allied Health Professionals' Role
Technical Monitoring
Allied health professionals, such as ophthalmic technicians, are responsible for the technical monitoring of the specular microscopy equipment. They ensure that the device settings are appropriate for each patient and that the images are high-quality for accurate assessment.[94]
Follow-up Tests
Based on the results from specular microscopy, they may conduct or recommend additional tests to gather comprehensive data on the patient's corneal health.[95]
Interprofessional Team Role
Collaborative Review
The interprofessional team collaborates in reviewing the data from specular microscopy. Ophthalmologists, nurses, and technicians can discuss the findings and implications for the patient's care plan.[96]
Continuity of Care
Coordinated efforts are made to schedule and monitor follow-up appointments and additional examinations or treatments as needed based on specular microscopy results.[4]
Outcome Assessment
The team periodically assesses the outcomes of patients who have undergone specular microscopy to ensure that any necessary adjustments to treatment or follow-up are made.[78]
Quality Assurance
The interprofessional team is involved in quality assurance, ensuring that specular microscopy and other diagnostic procedures are performed according to the highest standards and contribute positively to patient outcomes.[97]
Education and Training
Continuous education and training for all team members involved in specular microscopy are vital for maintaining a high standard of care. This may include reviewing the latest research, technology updates, and best practices.[98]
Communication
Effective communication channels among nursing, allied health, and the rest of the healthcare team are crucial for the timely sharing of information regarding patient status and monitoring findings from specular microscopy. The collective efforts of nurses, allied health professionals, and the broader interprofessional team are essential for the effective monitoring and management of patients who undergo specular microscopy, ensuring that the procedure provides valuable diagnostic information and contributes to high-quality patient care.[99]