Continuing Education Activity
Trichodysplasia spinulosa is a rare dermatologic condition characterized by painless erythematous papules on the face with folliculocentric keratin spines. This condition is unique to patients with trichodysplasia spinulosa-associated polyomavirus (TSPyV) in the setting of immunosuppression. The comparatively low incidence of trichodysplasia spinulosa within the susceptible population does not align with the ubiquitous nature of TSPyV in the general population, implying an insufficient understanding of this condition. This activity reviews the evaluation and proposed treatment of trichodysplasia spinulosa and highlights the role of the interprofessional team in identifying patients with this condition to improve patient care.
Objectives:
- Describe the epidemiology of trichodysplasia spinulosa.
- Review the clinical presentation of trichodysplasia spinulosa.
- Identify the diagnostic findings and histopathologic features of trichodysplasia spinulosa.
- Summarize the role of the interprofessional healthcare team in recognition, work-up, and treatment of this condition.
Introduction
Trichodysplasia spinulosa is a rare dermatologic condition characterized by painless erythematous papules on the face with folliculocentric keratin spines. This condition is unique to patients with trichodysplasia spinulosa-associated polyomavirus (TSPyV) in the setting of immunosuppression.[1]
The first report of this condition was in 1995, described as a disseminated follicular spiny hyperkeratosis, thought to be a side effect of cyclosporin.[2]
Subsequent case reports clarified that an immunosuppressed state, as opposed to cyclosporin specifically, is the common thread between patients with this condition. In 1999, trichodysplasia spinulosa was formally named as such with the discovery of its viral etiology, which in 2010 was characterized as a novel polyomavirus and aptly named trichodysplasia spinulosa-associated polyomavirus.[3][4] Ongoing recognition and reporting of trichodysplasia spinulosa in the medical literature are needed to better understand and treat this condition.
Etiology
Trichodysplasia spinulosa-associated polyomavirus (TSPyV) is classified under the family: Polyomaviridae, genus: Alphapolyomavirus, and species: Human polyomavirus 8.
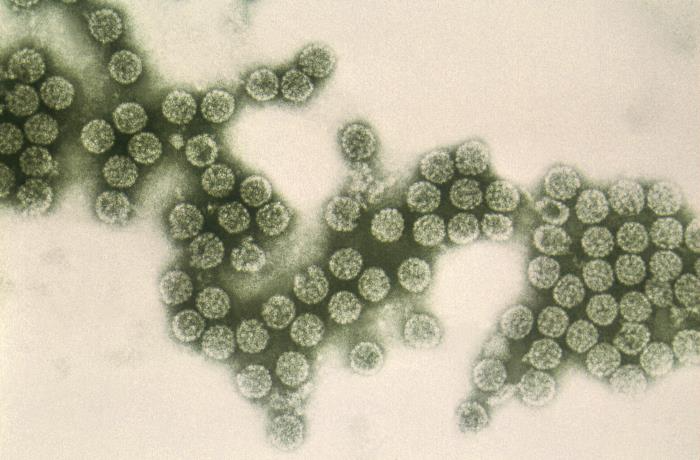
Polyomaviruses are about 45 nanometers in size, with an icosahedral capsid enclosing a circular, double-stranded DNA genome.[5] The name “polyomavirus” is derived from the prefix poly, meaning multiple, and the suffix oma, meaning tumor, based on the ability of certain polyomaviruses to induce tumor formation in immunocompromised animals.[6]
Human polyomaviruses are ubiquitous in the human population but typically asymptomatic.[5] Proposed mechanisms for trichodysplasia spinulosa in the context of immunosuppression include primary TSPyV infection, re-exposure to TSPyV, or reactivation of latent TSPyV similar to other well-known human polyomaviruses, JC virus, and BK virus.[7] The discordance between the high seroprevalence of TSPyV and the low number of reported cases of trichodysplasia spinulosa in the susceptible population implies other etiologic factors at play that have yet to be elucidated.
Epidemiology
Although trichodysplasia spinulosa as a clinical entity is predominantly documented in patients with compromised immune systems, infection with the causative virus is widespread. Serologic studies of individuals both with and without compromised immune systems indicate that the prevalence of TSPyV is between 65-80% in both populations. TSPyV has no apparent gender predilection, but seroprevalence differences between age groups implicate that primary infection occurs during childhood.[8]
Due to the rarity of this condition and the limited number of case reports, there is insufficient data to establish valid patterns of geographic distribution. Presumably, as this condition becomes more widely recognized and more frequently reported, geographic patterns may align with the distribution of immunocompromised populations.
Risk factors for trichodysplasia spinulosa include solid organ transplant, immunosuppressive medications, hematolymphoid malignancy, and HIV infection. All but one of the reported cases thus far have occurred in patients with indisputable immune compromise.
In September 2014, a case of trichodysplasia spinulosa was reported in a man with Gorlin syndrome being treated for multiple basal cell carcinomas with vismodegib. His trichodysplasia spinulosa eruption was reportedly painful, which is atypical, and resolved when vismodegib was discontinued. No historical or laboratory evidence of immunodeficiency was found.[9] Outliers aside, the majority of trichodysplasia spinulosa cases in the literature involve patients with solid organ transplants or leukemia.
Pathophysiology
The genomes of Polyomaviridae are divided into three main regions: the non-coding control region, the early gene region, and the late gene region. The non-coding control region, as the name implies, does not encode any genes to be transcribed but is responsible for the regulation of gene expression. The early gene region encodes proteins (large tumor antigen and small tumor antigen) that disrupt cell cycle control and enable viral genome replication utilizing host replication machinery. The late gene region encodes structural proteins (VP1, VP2, and VP3) that form the viral capsid and facilitate host cell attachment. Additional TSPyV proteins have been identified, but their functions remain unclear.[10] Other features of the polyomavirus genome include alternative splicing to allow multiple proteins to be encoded within a single transcript and the utilization of host cell histones to add structure and resilience to viral DNA.[6]
Serologic studies of TSPyV antibodies over time suggest that newborns initially have maternally sourced antibodies that wane over the following months. Throughout childhood, TSPyV transmission likely occurs between family members due to close interpersonal contact.[11] The precise route or mechanism of transmission of TSPyV is not yet known, nor is the duration of latent infection or preferred cellular reservoir. Aside from the skin, TSPyV has been detected in several different tissue types and bodily locations with unclear clinical relevance as there is no indication of TSPyV causing any other disease. Potential reservoir sites include the conjunctiva, nasopharynx, tonsils, upper respiratory tract, heart, gastrointestinal tract, cerebrospinal fluid, urine, and stool.
Hypotheses about the potential pathophysiology of trichodysplasia spinulosa are as follows: symptomatic reactivation of latent TSPyV due to immunosuppression, repeat exposure to TSPyV while immunosuppressed, and fulminant primary infection with TSPyV while immunosuppressed.[12]
As stated previously, there are likely unidentified variables besides immunosuppression and viral infection which influence the mechanism of disease. At the cellular level, one possible difference between asymptomatic and symptomatic TSPyV infection could be the route of intracellular viral transmission. The lytic pathway for intracellular viral transmission is a highly inflammatory process, whereas non-lytic transmission utilizing extracellular vesicles is more covert and, in fact, anti-inflammatory due to phosphatidylserine lipids on the vesicle surface.[13]
Although not specifically demonstrated with TSPyV, other polyomaviruses (BKPyV and JCPyV) have been shown to infect target cells by way of extracellular vesicles. Therefore, it is possible that other polyomaviruses harness this ability as well.[14]
Regardless of the mechanism by which this typically sub-clinical virus becomes pathogenic, once such conditions are met, inner root sheath follicular keratinocytes appear to be the primary target of TSPyV. Interactions with tumor suppressor genes pRB, p16, p21, and protein phosphatase 2 lead to hyperproliferation of the inner root sheath, presumably producing the characteristic follicular spines.[15][16]
The absence of dermal papillae in affected skin explains the associated alopecia because papillae are essential for new hair growth. For this same reason, the mechanism by which the papillae are lost must be reversible because hair regrows after the condition resolves.[17][18] Ultimately, further research is needed to fully understand and define the pathophysiology of trichodysplasia spinulosa.
Histopathology
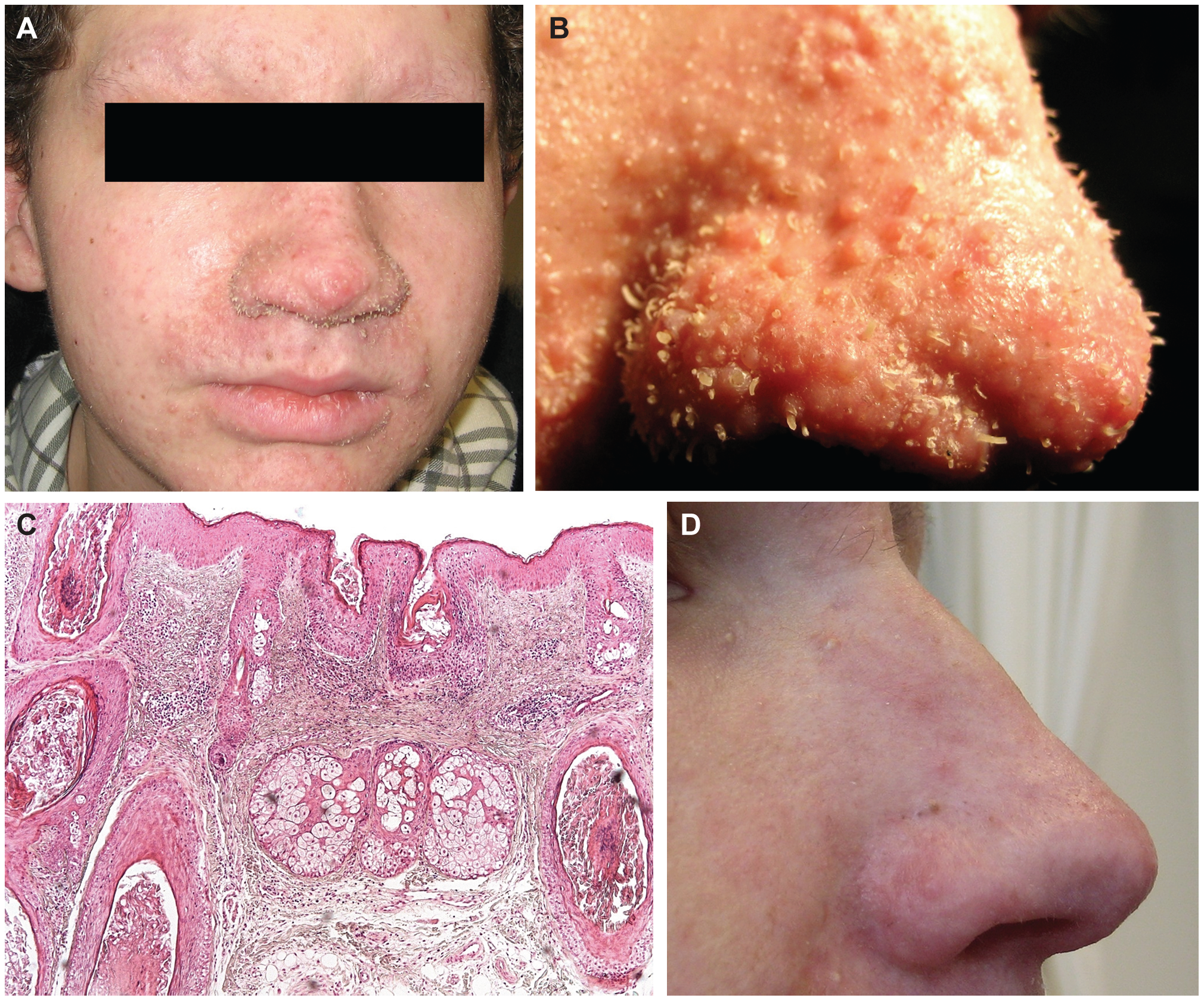
Key histologic features include dilated hair follicles, keratin plugging, abundant dystrophic and cornfield inner root sheath epithelium, and numerous, large eosinophilic trichohyalin granules within the inner root sheath cells.
Other features include apoptotic cells, epidermal acanthosis and may include mild perifollicular lymphocytic inflammation.[3][5] Hair shafts and papilla are notably absent.[17]
The inner root sheath epithelial cells stain with Ki-67, indicative of hyperproliferation, and toluidine blue, which reflects increased nucleic acids from dysplastic cells.[5][19] The abundance of viral DNA in affected tissue may also contribute to toluidine blue staining based on its affinity for nucleic acids. Still, this extrapolation is not corroborated by dermatopathology texts, potentially due in part to the rarity of this condition.
History and Physical
Patients with trichodysplasia spinulosa present with a papular, follicular facial rash with spiny keratosis, referred to as “spicules,” and non-scarring alopecia of the eyebrows. The lesions are often asymptomatic but may be somewhat pruritic. As lesions coalesce and the skin thickens, facial features can become distorted and cause a leonine facial appearance. Papules may be flesh-colored, erythematous, or hyperpigmented. The central face is most frequently affected, but other areas such as the ears, trunk, and arms may also be involved.[3][17]
Immunosuppression is a critical historical component, whether it is due to medications, organ transplant, cancer, or HIV. Most reported cases involve patients with solid organ transplants on anti-rejection medications or patients being treated for hematolymphoid malignancy.
Evaluation
In addition to histopathological examination of a skin biopsy, diagnostic tools for trichodysplasia spinulosa may include polymerase chain reaction or electron microscopy. Serologic tests are not necessary to establish the diagnosis of trichodysplasia spinulosa. Still, they may be indicated if an underlying immune-compromising condition has not previously been identified for any patient presenting with trichodysplasia spinulosa.
Polymerase chain reaction: PCR is considered the “gold standard” diagnostic test for trichodysplasia spinulosa when used in concert with clinical observation and histology. Utilizing a tissue sample of the affected skin or spicules removed from the affected skin, quantitative PCR demonstrates high copy numbers of the TSPyV genome. Low copy numbers and samples from unaffected areas are not diagnostically useful as TSPyV has been identified in various tissues types, stated previously, to include individuals with no clinical evidence of trichodysplasia spinulosa.[5]
Electron microscopy: On transmission electron microscopy, the affected tissue will have intranuclear clusters of icosahedral viral inclusions with a “bumpy” appearance, around 40 nanometers in size.[3][17]
Serology: For diagnostic purposes, serology does not have significant utility based on the ubiquitous nature of this virus, as discussed previously. However, given the clear correlation between compromised immunity and this condition, any patient without a prior diagnosis should undergo a thorough history and physical. Further testing for immunodeficiency should be guided by clinical judgment but may include a complete blood count, HIV test, peripheral blood smear, or antibody titers.
Treatment / Management
Several treatments for trichodysplasia spinulosa have been attempted, but a widely accepted treatment plan still evades the medical community. The three seemingly most effective approaches include topical cidofovir, oral valganciclovir, and reduction of immunosuppression.
Topical cidofovir 3% has been recommended as a first-line treatment for trichodysplasia spinulosa based on a trend of treatment success in case reports. Topical cidofovir as a possible treatment is further supported by in vitro evidence that cidofovir inhibits human polyomavirus replication. Oral valganciclovir has also been effective in several cases and is more accessible than topical cidofovir but lacks the evidentiary support of in vitro or in vivo studies showing activity against human polyomavirus. Regarding reducing immunosuppressive therapy, the risk of sub-therapeutic dosing, transplant rejection, and progression or relapse of underlying disease often outweighs the benefit of reversing trichodysplasia spinulosa.
Various other topical treatments have been tried with inconsistent efficacy, such as leflunomide, topical retinoids, steroids, imiquimod, keratolytics, emollients, green tea extract, and other anti-virals.[1]
Differential Diagnosis
Histopathology and polymerase chain reaction can be used to definitively diagnose trichodysplasia spinulosa and rule out the other clinically similar conditions listed below.
- Trichostasis spinulosa: usually asymptomatic, dilated hyperkeratotic follicles with several vellus hairs.[20]
- Follicular hyperkeratotic spicules: predominantly associated with multiple myeloma and located on the face.
- Lichen spinulosus: follicular papules, keratin spines, favors trunk and extremities.
- Spiky follicular mycosis fungicides: erythematous, hyperkeratotic follicular papules associated with lymphoma.[21]
- Disseminated spiked hyperkeratosis: small, non-follicular keratin spikes with a genetic association.[22]
- Ulerythema ophryogenes (keratosis pilaris atrophicans fasciei): erythematous facial papules, scarring alopecia of eyebrows, permanent eyebrow loss.
- Follicular-based graft versus host disease: hyperkeratotic, erythematous, folliculocentric papules, occurs post-bone marrow transplant.[3][23]
Other polyomaviruses of the skin: Seven different species of polyomavirus have been detected in the skin. In addition to TSPyV, Merkel cell polyomavirus (MCPyV) and human polyomaviruses 6, 7, 9, 10, and 13 have been isolated from the skin. However, only MCPyV, HPyV6, and HPyV7 have pathologic associations at this time, particularly in the immunocompromised setting. MCPyV causes Merkel cell carcinoma, classically described as a painless, rapidly growing, reddish-blue nodule. HPVy6 and HPVy7 are associated with pruritic and dyskeratotic dermatosis, described as pruritic, hyperpigmented, lichenified plaques on the torso and extremities. However, case reports are limited such that declaring a “classic” presentation is premature.[24]
Prognosis
Although there is no widely accepted treatment regimen for trichodysplasia spinulosa, the prognosis is relatively good, although it may take months to years to fully resolve. In some cases, the condition regresses spontaneously or after the completion of the immunosuppressive treatment. The associated alopecia of the eyebrows and eyelashes and leonine facial disfiguration also resolve over time with the resolution of active disease. The underlying condition that either causes or requires immunosuppression is much more pertinent to the prognosis for the patient as a whole.
Complications
Potential complications include pruritus, temporary loss of eyebrows and eyelashes, facial disfiguration, and the associated psychological distress that accompanies these symptoms.
Deterrence and Patient Education
Patients should be reassured that trichodysplasia spinulosa is benign, and with time, treatment, and, if applicable, completion of immunosuppressive therapy, the skin changes will resolve, and eyebrows will regrow. One specific treatment has not yet been widely accepted; however, topical cidofovir and oral valganciclovir are reasonable options with multiple reports of success.[1]
Enhancing Healthcare Team Outcomes
Thankfully, there is no known mortality attributed to trichodysplasia spinulosa, and the morbidity may be primarily cosmetic. Still, it has the potential to cause pruritus, psychological stress associated with its prominently visible symptoms, and psychological stress associated with prognostic uncertainty while patients await a definitive diagnosis and treatment plan. The two known contributing factors for trichodysplasia spinulosa (TSPyV infection and immunosuppression) are not reasonable targets for prevention due to the ubiquitous nature of TSPyV and the implied medical necessity of immunosuppressive therapy in the relevant patient population.[1]
However, other contributing factors yet to be known could theoretically provide an appropriate avenue for prevention. Collaboration between an interprofessional team of dermatologists, immunologists, virologists, and other healthcare specialists in both clinical and academic settings is required to enhance patient-centered care. Clinical recognition and publication of cases of trichodysplasia spinulosa are needed to better understand risk factors and build the repertoire of treatment options. Investigation of the pathophysiology of this condition and mechanisms of proposed treatments is necessary to clarify and validate their efficacy. Ultimately, expanding upon the paucity of knowledge about trichodysplasia spinulosa will increase diagnostic fidelity, improve patient counseling and reassurance, and firmly establish effective treatment options. [Level 5]