Introduction
Glaucoma is a chronic progressive optic neuropathy characterized by visual field changes and optic nerve cupping. It is the second most common cause of irreversible blindness across the globe.[1] Managing glaucoma in developed and developing countries poses a substantial economic and social burden. Glaucoma accounts for a total of 8% percent of blindness worldwide.[2] The modifiable risk factor for glaucoma is intraocular pressure (IOP) which is the main line of treatment.[3]
The conventional treatment modalities for mild to moderate glaucoma are antiglaucoma medications and laser procedures in the form of laser iridotomy and laser trabeculoplasty. Advanced cases or cases with non-resolving intraocular pressure require a target IOP to safeguard vision and field. The treatment for advanced stages can be trabeculectomy and glaucoma drainage devices (GDD).[4]
Scientists and Ophthalmic researchers have always searched for alternative surgical treatment modalities for rapid and effective reduction of IOP, maintaining a safety profile, using ab-interno procedures with minimal tissue trauma and faster recovery.[5] These procedures have been labeled microinvasive or minimally invasive glaucoma surgery (MIGS).[6]
MIGS work either by bypassing the trabecular meshwork (Hydrus stent, iStent, Trabectome, Kahook dual blade), increased aqueous outflow through Schlemm's canal (canaloplasty), increased uveoscleral outflow (iStent supra), aqueous shunt through subconjunctival space (XEN implant) or by ciliary body ablation (endocyclophotocoagulation).[7]
Although long-term results are awaited, MIGS has revolutionized glaucoma management with minimal tissue trauma, good post-operative recovery, and improved patient satisfaction. The MIGS options have opened a whole new bunch of options for all the glaucoma specialists with promising results. Another advantage is that MIGS can be combined with phacoemulsification reducing surgical time. This activity deals with various available MIGS options, their design, efficacy, the technique of implantation, and safety profile for better patient management.[8]
MIGS Characteristics
- High safety- reduced risk of complications like choroidal detachment, hypotony, hemorrhage, and effusion
- Minimal alteration of normal angle anatomy- MIGS improves the physiological aqueous outflow by minimal disruption to angle structures
- Ab interno procedure- MIGS is performed through the clear corneal incision using an ab interno approach with direct visualization of the anatomical target
- MIGS is an excellent alternative to traditional angle surgery in lowering the IOP - the amount of IOP reduction is lesser than trabeculectomy but should be at least 20%.
- Good post-operative recovery with minimal bedtime for patients - MIGS offers ease to patients as well as surgeons[8]
Minimally Invasive Glaucoma Surgery Classification
There are four main approaches for IOP reduction by MIGS
1. Increasing Aqueous Outflow from Trabecular Meshwork and Schlemm Canal
Stents
- iStent
- iStent Inject
- Hydrus
Tissue Removal
- Gonioscopy-assisted transluminal trabeculotomy (GATT)
- Kahook dual blade goniotomy
- Trabectome
- TRAB 360 system
- Excimer laser trabeculostomy
Through Schlemm Canal
- VISCO360 device
- Ab Interno canaloplasty (ABiC) using the iTrack microcatheter system[9]
2. Increased Uveoscleral Outflow through Suprachoroidal Space
3. Aqueous Shunt through Subconjunctival Space
4. Ciliary Process Ablation Resulting in Reduced Aqueous Outflow
- Endocyclophotocoagulation[12]
Table Depicting Various Types of MIGS Based On Mechanism of Action
|
S. No
|
MIGS Mechanism
|
Type of MIGS
|
Remarks
|
|
1
|
Increased Trabecular Outflow
|
iStent Micro-Bypass
|
Made of titanium, the stent has a heparin coating and is non-ferromagnetic. Dimensions 1 mm x 0.3 mm. Ab interno insertion into Schlemm's canal.
|
|
|
|
Gonioscopy-assisted transluminal trabeculotomy (GATT)
|
In this technique microcatheter or prolene/ nylon suture is passed through Schlemm's canal 360 degrees under gonioscopic assistance. This is an ab interno trabeculotomy.
|
|
|
|
Trabectome
|
This is an ab interno technique which is a combination of electrocautery, irrigation, and aspiration.
|
|
|
|
TRAB 360 Trabeculotomy
|
This is performed with the help of a disposable, non-powdered device. A flexible nylon trabeculotome is advanced in the Schlemm canal 180 degrees and then lysed.
|
|
|
|
Kahook Dual Blade
|
This is a type of ab interno trabeculotomy which is done using a stainless steel blade.
|
|
|
|
Ab interno canaloplasty
|
This is a microcatheter used to vasodilate the Schlemm's canal.
|
|
|
|
Hydrus Microstent
|
This is a crescent-shaped ab interno nickel-titanium device inserted into the Schlemm canal.
|
|
2
|
Increase Uveoscleral / Suprachoroidal/ Supraciliary Outflow
|
CyPass Micro-Stent
|
This stent has fenestrations made up of biocompatible polyamide material—inserted ab internally between the sclera and suprachoroidal space.
|
|
|
|
iStent Supra
|
This is a heparin-coated stent with a polyethersulfone and titanium sleeve inserted internally between the sclera and suprachoroidal space.
|
|
3
|
Increase Subconjunctival Outflow
|
XEN Glaucoma Treatment System
|
This is a tube implant made up of gelatin and glutaraldehyde. Inserted ab internally into the anterior chamber, then through the sclera into subconjunctival space, forming a bleb.
|
|
|
|
InnFocus MicroShunt (PRESERFLO MicroShunt)
|
It is a flexible micro shunt composed of SIBS (poly(styrene-block-isobutylene-block-styrene)) inserted ab externally into the anterior chamber through subconjunctival space, forming a bleb.
|
|
4
|
Decrease Aqueous Production
|
Endocyclophotocoagulation
|
Cyclodestruction of the ciliary body using continuous mode energy
|
Technique or Treatment
Trabecular Meshwork Bypass
The juxtacanalicular trabecular meshwork is the site of greatest resistance to aqueous outflow. Through the technique of MIGS, the tissue can be ablated or excised, or trabecular meshwork can be bypassed through stents, thus allowing direct bypass of aqueous humor into the Schlemm's canal.[19]
This is indicated in POAG, PXFG, pigmentary glaucoma, and ocular hypertension with a target IOP of 15 to 16 mm Hg. TM bypass is contraindicated in PACG, NVG, angle dysgenesis, corneal opacity, and elevated episcleral venous pressure cases like Sturge Weber syndrome.[20] The complications associated with TM bypass include hyphema, stent displacement, malposition, obstruction, peripheral anterior synechiae, and transient IOP elevation.[21]
Stents
iStent
iStent is the first FDA-approved MIGS that came on the market in 2012. This is a very small stent coated with heparin and made of titanium non-ferromagnetic material. This is a ridge shaped like a snorkel.[22]
The stent measures 1 mm x 0.3 mm, and the snorkel measures 0.25 mm in height with a central lumen of 120 um, which will project in the anterior chamber. The device is designed to have three arches for retention that ensure its proper placement in the Schlemm's canal at the nasal angle. iStent is implanted with the help of an ab interno approach using a pre-loaded inserter through a clear corneal incision under gonioscopic guidance.
iStent implantation results in an IOP reduction of approximately 25% in nearly 70% of the patients, significantly reducing the medication burden. Single iStent placement may result in an IOP reduction of 23 to 27%, whereas multiple iStent may cause a 40 to 44% reduction in IOP in approximately 60% of patients by one year, as per previous studies.[23]
iStent Inject
This device was FDA approved in 2018 and is a second-generation TM bypass stent. This is considered to be a small medical implant in the human body. Like iStent, this is heparin-coated and non-ferromagnetic, made up of titanium. The stent dimensions are the height of 360 um and 230 um in diameter with a central lumen of 80 microns.[24]
The stent has a head with the tapering end that sits in the Schlemm's canal, and the thorax enters the TM and anterior chamber. iStent inject is inserted by ab interno approach through gonioscopic visualization.[22]
The device is available as a pre-loaded injector (G2-M-IS injector system) with two stents placed nasally in the TM and Schlemm canal 30 to 60 degrees apart. Fea et al., in their analysis, documented a reduction in IOP of 35 to 39% with a decrease in medication load, and 65 to 75% of patients were free of medications at one year. One of the previous studies showed that the IOP reduction by implantation of two stents is equivalent to IOP reduction with two antiglaucoma drugs after one year.[8]
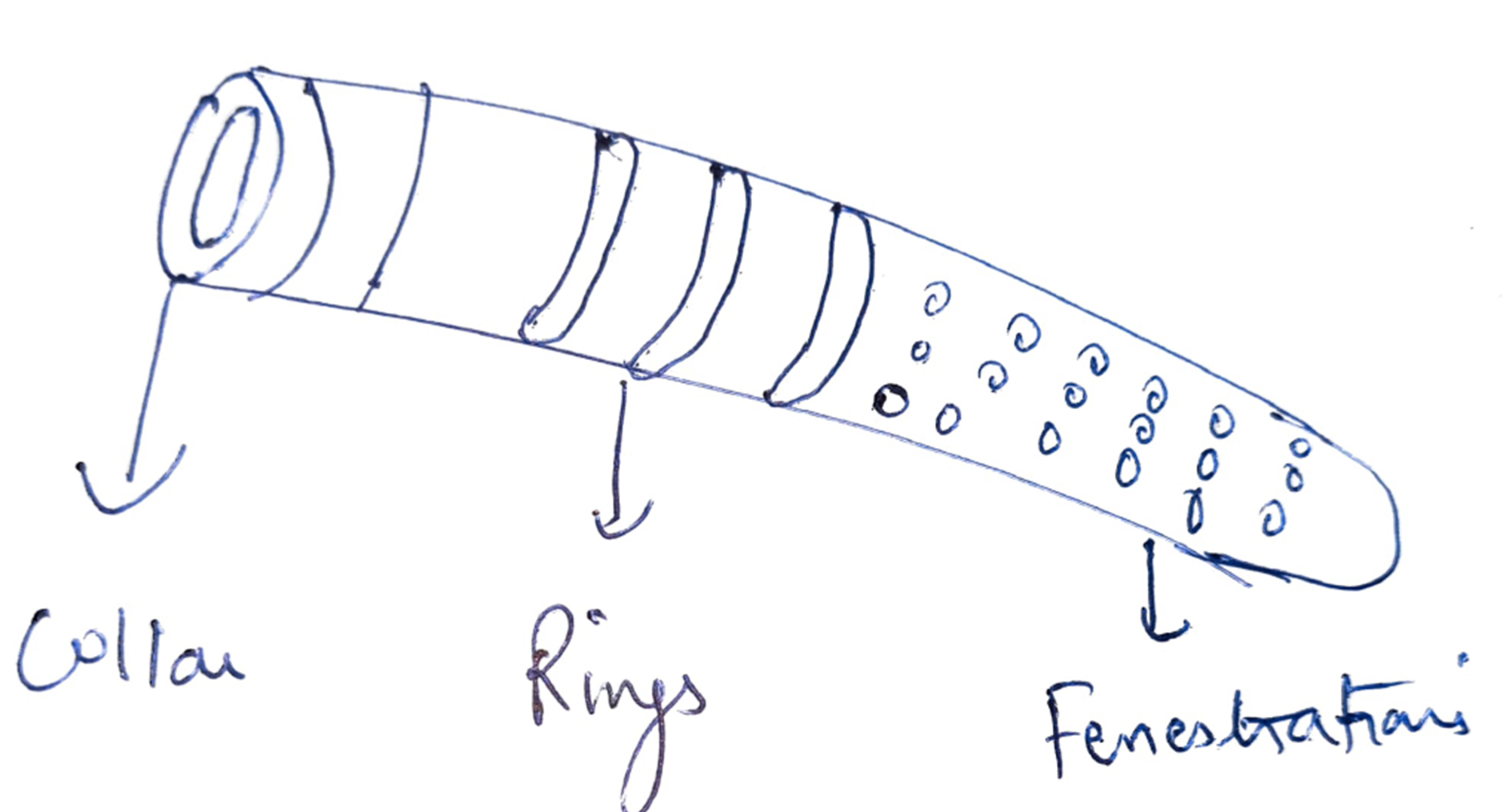
Hydrus Stent
This stent is a small crescent-shaped stent of 8 mm in length and 290 um in diameter. This has a small inlet and three window-shaped openings which sit in the anterior chamber. This stent is made up of nitinol material, which has good shaping memory. The device can be implanted in the nasal or inferior temporal quadrant through a pre-loaded injection using a clear corneal incision.[25]
The device has small intracanalicular scaffolds which occupy nearly three clock hours of Schlemm's canal and spare the collector channel ostia in the posterior portion of the canal. This stent works on the unique trimodal mechanism. It bypasses the trabecular meshwork and allows aqueous flow. It dilates the Schlemm's canal 4 to 5 times, making outflow through collector channels easier. The HORIZON study concluded that there was more than a 20% reduction in IOP in 80% of the cases, and 73% of eyes were free of antiglaucoma medications at 24 months.[26]
Another study concluded a 20% IOP reduction in 77.3% of eyes at 24 months post-surgery compared to antiglaucoma medications. The COMPARE study compared Hydrus vs. iStent and concluded that 39.7% of eyes showed more than 20% IOP reduction than 13.3% of eyes in the iStent group. Approximately 46.6% were medication-free in the Hydrus group compared to 24% in the iStent group.[27]
Tissue Excision (Trabeculotomy)- Bypassing the TM
Kahook Dual Blade (KDB) Goniotomy
This is a stainless steel disposable dual-side blade having a sharp tip. The ends are tapered for a smooth entry through the trabecular meshwork. The blade's heel snugs easily within the Schlemm's canal for smooth access, and the front part of the blade generates stretch. The double blade makes parallel incisions in the TM. This helps in the total removal of the TM with minimal tissue damage and less fibrosis over time, giving a long-term outcome.[28]
The blade is used by the ab interno approach under gonioscopic visualization. Previous studies have documented approximately 20% reduction in IOP in 70% of eyes with at least one medication with KDB at one-year follow-up. Good results are seen in patients with pseudoexfoliation glaucoma, pigmentary glaucoma, and secondary open-angle glaucoma, as it helps remove pigments from the TM. KDB has also been shown to be beneficial in congenital glaucoma; and advanced and refractory glaucoma cases.[29]
Trabectome
The trabectome was invented by Baerveld, Chuck, and Irvine and got FDA approval in 2004 to manage adult and pediatric cataracts. This comprises a handpiece of 19.5 gauzes, automated irrigation and aspiration, electronic cautery, and a foot pedal to control the irrigation and aspiration.[30]
The handpiece's footplate stretches the TM and protects the surrounding tissue from the thermal burn. The handpiece tip also has a bipolar cautery which generates plasma at 550 kHz, which ablated the TM by creating ionizing molecules. This creates an opening from AC into the Schlemm canal and collector channels. Trabectome is documented to reduce IOP by approximately 40%. It has also been proven effective in narrow-angle glaucoma, failed trabeculectomy, shunts, and refractory glaucoma cases.[31]
Laser Trabeculostomy
This was first described by Berlin in 1987 using an excimer laser and used by Vogel and Lauritzen in 1997. This is performed using a fiber optic delivery system and a short pulse of 80ns, 308 nm xenon chloride laser to the TM. The energy pulse delivered is 1.2 mJ, and the pulse duration is 10 to 60 ns with a frequency of 20 Hz. This procedure helps remove the tissue obstructing the aqueous outflow with significantly less damage to the surrounding tissue. A total of 10 microchannels over 90 degrees are created, 500 um apart.
This process is also labeled pneumatic canaloplasty as it dilates the Schlemm canal and collector channels. LT is also an ab interno procedure performed under direct visualization of gonioscope. This process has a moderate reduction of IOP between 20 to 40%.[32]
Gonioscopy Assisted Transluminal Trabeculotomy (GATT)
This was first described by Grover et al. in 2014. This consists of a microsurgical forceps and an iTrack microcatheter with a 200 um diameter shaft having a lubricating coating and a distal tip with illumination to monitor the catheter location. In this ab interno approach, the catheter or suture is passed through the nasal quadrant under gonioscopic visualization into the Schlemm canal and then advanced to 360 degrees with the help of micro-forceps.[33] Then traction is applied to break the TM, thus resulting in 360-degree trabeculectomy. GATT has shown to be effective in approximately 70 to 90% of cases compared to the ab externo approach with an IOP reduction of 30 to 40%. GATT was primarily used for congenital glaucoma and juvenile open-angle glaucoma. This is an essential option for refractory glaucoma and posts trabeculectomy cases. GATT is contraindicated in patients who require anticoagulants or have bleeding diatheses.[34]
Ab Interno Canaloplasty (AbiC)
This is performed with the help of the iTrack microcatheter. The catheter is 200 um thick with a bulbous 250u tip and has a lubricating coating for smooth passage, viscoinjector, and fiber optic enabled illumination. The microcatheter is passed through Schlemm's canal. Then it i's removed as the Visco surgical device is inserted to Visco dilate all the sites of resistance outflows, such as the Schlemm canal and collector channels. Visco-dilatation helps to create perforations within the TM, which increase aqueous outflow. This technique results in 30 to 40% IOP reduction at 12 months with less than two antiglaucoma drugs.[35]
Suprachoroidal Shunts
These shunts help direct the aqueous outflow to the suprachoroidal space in a controlled manner and thus increase the uveoscleral outflow. This acts as a permanent reservoir between the anterior chamber and supraciliary space.[36]
Cypass Stent
They were first used in 2016 and FDA approved for glaucoma management. The stent is a polyamide stent, flexible, fenestrated, micro-sized of 6.35 mm x 510 um, having a lumen of 300 um. This is available as a pre-loaded device with a guidewire confirming the shape of the sclera to help dissection and insertion between the anterior chamber/ sclera and suprachoroidal space. The device results in a higher rate of endothelial cell loss; hence was withdrawn from the market in 2018.[37]
iStent Supra
This is a curved heparin-coated tube with a lumen of 0.16 mm. It is made up of biocompatible polyether sulfone consisting of a titanium sleeve and retention ridge to hold it in place. The stent is mounted with a guide so that it can be easily inserted in the anterior chamber and suprachoroidal space by ab interno approach under gonioscopic guidance. iStent supra, along with Travoprost, is shown to reduce IOP by 20% in patients with advanced glaucoma.[38]
Subconjunctival Filtration Stents
Xen Implant (Xen Gel Stent)
This subconjunctival implant allows the aqueous flow to the subconjunctival space. This is a kind of bleb-forming procedure like trabeculectomy. Xen implant is implicated for open-angle glaucoma in moderate to an advanced stage, refractory glaucoma; post failed trabeculectomy, phakic or pseudophakic glaucoma. The device is contraindicated in angle-closure glaucoma, conjunctival scarring, neovascularization, inflammation, and silicone oil or vitreous in the anterior chamber.[38]
This hydrophilic tube is made up of porcine collagen-derived gelatin, which is crosslinked with glutaraldehyde. The device is in a rigid state and straight when dry and mouldable when soft and hydrated. This is 6 mm in length and has three types XEN 140, XEN 63, and XEN 45. XEN 45 is FDA-approved with an inner lumen of 45 um and an outer diameter of 150 um. The device has the advantage that it prevents the risk of hypotony. This is available as a pre-loaded device with a disposable 27G injector inserted in the anterior chamber through an ab interno approach.[39]
The device is placed in the superonasal or superior quadrant and is passed through the sclera to be placed in the subconjunctival space. There is a bleb formation at the end of the procedure, and mitomycin C can be used to reduce fibrosis. The Xen gel implant can migrate into the anterior chamber or gape through the conjunctiva, or there may be fragmentation of the stent. Hypotony, hyphema, and stent obstruction can also occur. Xen gel implant causes a 29 to 41% reduction in IOP in nearly 45% of patients without antiglaucoma drugs.[40]
InnFocus MicroShunt (PRESERFLO MicroShunt)
This is an 8.5 mm long micro shunt with a 1 mm fin that divides the shunt into a long proximal (4.5 mm) and short distal (3 mm) part. The material is poly (styrene-block-isobutylene-block-styrene) or SIBS. This material has been used for coronary stents and is biologically inert. The external lumen is 350 um, and the internal lumen is 75 um. The placement of the device requires fornix-based peritomy, dissection of attachments of the tenon, and application of mitomycin-c (MMC) soaked sponges to the scleral bed. After washing with saline, a point 3 mm behind the limbus is marked through which the device is entered intra-sclerally to open inside the anterior chamber parallel to the iris. The tenon and conjunctiva are sutured after confirming the patency of the device.
Batlle and colleagues showed that the IOP was controlled within low teens in most patients three years after receiving the shunt with MMC.[41] Most complications, including transient hypotony and transient choroidal effusion, resolved spontaneously.[41] In a retrospective study on 164 eyes, complete success and qualified success were achieved at one year in 76.9% and 92.5% of eyes, respectively.[42]
Endocytophotocoagulation (ECP)
This results in decreased aqueous production by ciliary body ablation and, hence, a reduction in IOP. The mechanism is localized ciliary process shrinkage with temporary occlusive vasculopathy. If the ciliary processes are visualized directly, it results in nominal collateral damage. This is indicated in mild to moderate glaucoma cases along with cataract extraction with sparing of the conjunctiva, refractory open-angle glaucoma cases, pediatric glaucoma, neovascular glaucoma, and contraindicated in active inflammation. Rarely ECP may result in zonular damage or cataract progression.[43]
The device is a probe with a laser unit having a diode laser. The wave energy is emitted at a wavelength of 810 nm with 175 W xenon light, helium-neon laser, and a video camera imaging system. The probe consists of a fiber optic system that transmits the signals. The probe can be inserted through a clear corneal incision and can be straight or curved. The video monitor allows the endoscopic view; thus, the surgeon can control the foot pedal to fire the laser. The laser power is between 0.2 to 0.25 watts, and the power is titrated to see blanching. At one moment, a 200 to 300-degree angle is treated.
In refractory glaucoma cases, 1 to 2 mm of pars plana is also coagulated to reduce the IOP aggressively; this is labeled as ECP plus. This is done through the pars plana approach and is preceded by pars plana vitrectomy. The adverse effects reported are secondary glaucoma, hyphema, post-operative inflammation, retinal detachment, choroidal detachment, cystoid macular edema, IOL (intraocular lens) dislocation, and capsular opacification. It results in approximately 35% IOP reduction in patients with one medication. Combined with phacoemulsification, ECP reduces IOP by 20 % in about 60% of patients, and a success rate of 43% is noted in pediatric patients.[44]