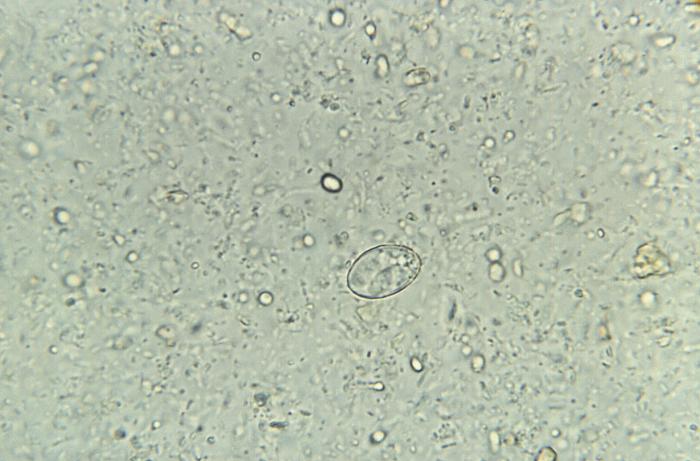
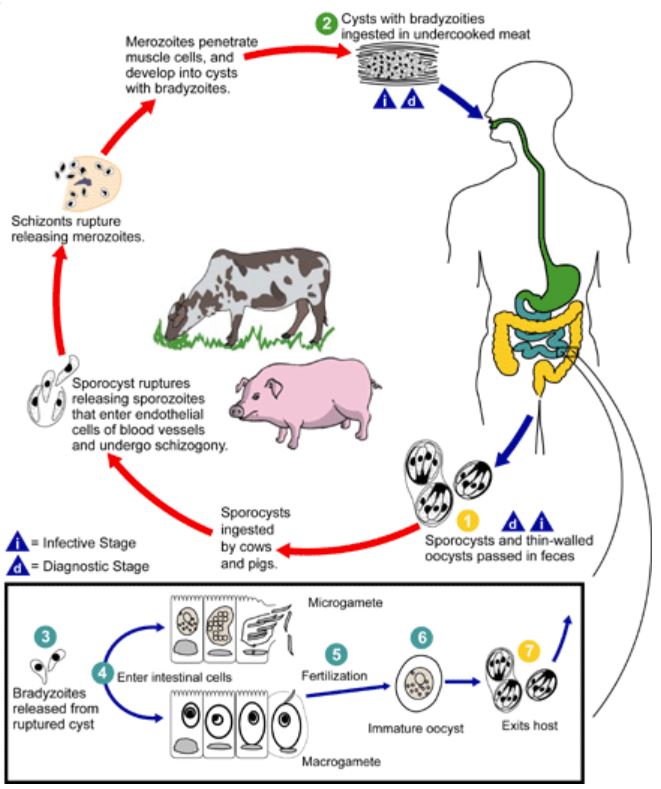
[1]
Fayer R. Sarcocystis spp. in human infections. Clinical microbiology reviews. 2004 Oct:17(4):894-902, table of contents
[PubMed PMID: 15489353]
[2]
Markus MB, Killick-Kendrick R, Garnham PC. The coccidial nature and life-cycle of Sarcocystis. The Journal of tropical medicine and hygiene. 1974 Nov:77(11):248-59
[PubMed PMID: 4219030]
[3]
Mohammad N, Besari AM, Nair PK, Wan Ghazali WS. Muscular sarcocystosis: an index case in a native Malaysian. BMJ case reports. 2017 Jul 26:2017():. pii: bcr-2017-220490. doi: 10.1136/bcr-2017-220490. Epub 2017 Jul 26
[PubMed PMID: 28747414]
Level 3 (low-level) evidence
[4]
Fayer R, Esposito DH, Dubey JP. Human infections with Sarcocystis species. Clinical microbiology reviews. 2015 Apr:28(2):295-311. doi: 10.1128/CMR.00113-14. Epub
[PubMed PMID: 25715644]
[5]
Beaver PC, Gadgil K, Morera P. Sarcocystis in man: a review and report of five cases. The American journal of tropical medicine and hygiene. 1979 Sep:28(5):819-44
[PubMed PMID: 114067]
Level 3 (low-level) evidence
[6]
Wong KT, Leggett PF, Heatley M. Apparent absence of Sarcocystis infection in human tongue and diaphragm in Northern Ireland. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993 Jul-Aug:87(4):496
[PubMed PMID: 8249098]
[7]
Thomas V, Dissanaike AS. Antibodies to Sarcocystis in Malaysians. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1978:72(3):303-6
[PubMed PMID: 97821]
[8]
Harris VC, van Vugt M, Aronica E, de Bree GJ, Stijnis C, Goorhuis A, Grobusch MP. Human Extraintestinal Sarcocystosis: What We Know, and What We don't Know. Current infectious disease reports. 2015 Aug:17(8):495. doi: 10.1007/s11908-015-0495-4. Epub
[PubMed PMID: 26115699]
[9]
Clavel A, Doiz O, Varea M, Morales S, Castillo FJ, Rubio MC, Gómez-Lus R. [Abdominal discomfort and soft stools in a habitual consumer of rare beef]. Enfermedades infecciosas y microbiologia clinica. 2001 Jan:19(1):29-30
[PubMed PMID: 11256244]
[10]
Yu S. [Field survey of sarcocystis infection in the Tibet autonomous region]. Zhongguo yi xue ke xue yuan xue bao. Acta Academiae Medicinae Sinicae. 1991 Feb:13(1):29-32
[PubMed PMID: 1831698]
Level 3 (low-level) evidence
[11]
Khieu V, Marti H, Chhay S, Char MC, Muth S, Odermatt P. First report of human intestinal sarcocystosis in Cambodia. Parasitology international. 2017 Oct:66(5):560-562. doi: 10.1016/j.parint.2017.04.010. Epub 2017 May 3
[PubMed PMID: 28476340]
[12]
Agholi M, Taghadosi Z, Mehrabani D, Zahabiun F, Sharafi Z, Motazedian MH, Hatam GR, Naderi Shahabadi S. Human intestinal sarcocystosis in Iran: there but not seen. Parasitology research. 2016 Dec:115(12):4527-4533
[PubMed PMID: 27637226]
[13]
Meloni BP, Thompson RC, Hopkins RM, Reynoldson JA, Gracey M. The prevalence of Giardia and other intestinal parasites in children, dogs and cats from aboriginal communities in the Kimberley. The Medical journal of Australia. 1993 Feb 1:158(3):157-9
[PubMed PMID: 8450779]
[14]
Makhija M. Histological identification of muscular sarcocystis: a report of two cases. Indian journal of pathology & microbiology. 2012 Oct-Dec:55(4):552-4. doi: 10.4103/0377-4929.107813. Epub
[PubMed PMID: 23455804]
Level 3 (low-level) evidence
[15]
Agarwal PK, Srivastava AN. Sarcocystosis in man: a report of two cases. Histopathology. 1983 Sep:7(5):783-7
[PubMed PMID: 6414925]
Level 3 (low-level) evidence
[16]
Chen X, Zuo Y, Zuo W. [Observation on the clinical symptoms and sporocyst excretion in human volunteers experimentally infected with Sarcocystis hominis]. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi = Chinese journal of parasitology & parasitic diseases. 1999:17(1):25-7
[PubMed PMID: 12563811]
[17]
Tappe D, Stich A, Langeheinecke A, von Sonnenburg F, Muntau B, Schäfer J, Slesak G. Suspected new wave of muscular sarcocystosis in travellers returning from Tioman Island, Malaysia, May 2014. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014 May 29:19(21):. pii: 20816. Epub 2014 May 29
[PubMed PMID: 24906376]
[18]
Poulsen CS, Stensvold CR. Current status of epidemiology and diagnosis of human sarcocystosis. Journal of clinical microbiology. 2014 Oct:52(10):3524-30. doi: 10.1128/JCM.00955-14. Epub 2014 Apr 23
[PubMed PMID: 24759707]
[19]
Tungtrongchitr A, Chiworaporn C, Praewanich R, Radomyos P, Boitano JJ. The potential usefulness of the modified Kato thick smear technique in the detection of intestinal sarcocystosis during field surveys. The Southeast Asian journal of tropical medicine and public health. 2007 Mar:38(2):232-8
[PubMed PMID: 17539271]
Level 3 (low-level) evidence
[20]
Italiano CM, Wong KT, AbuBakar S, Lau YL, Ramli N, Syed Omar SF, Kahar Bador M, Tan CT. Sarcocystis nesbitti causes acute, relapsing febrile myositis with a high attack rate: description of a large outbreak of muscular sarcocystosis in Pangkor Island, Malaysia, 2012. PLoS neglected tropical diseases. 2014 May:8(5):e2876. doi: 10.1371/journal.pntd.0002876. Epub 2014 May 22
[PubMed PMID: 24854350]
[21]
LAARMAN JJ. Isospora hominis (Railliet and Lucet 1891) in the Netherlands. Acta Leidensia. 1962:31():111-6
[PubMed PMID: 13927789]
[22]
Giboda M, Rakár J. First record of "Isospora hominis" in Czechoslovakia. Folia parasitologica. 1978:25(1):16
[PubMed PMID: 640517]
[23]
Bunyaratvej S, Bunyawongwiroj P, Nitiyanant P. Human intestinal sarcosporidiosis: report of six cases. The American journal of tropical medicine and hygiene. 1982 Jan:31(1):36-41
[PubMed PMID: 6800273]
Level 3 (low-level) evidence
[24]
Esposito DH, Stich A, Epelboin L, Malvy D, Han PV, Bottieau E, da Silva A, Zanger P, Slesak G, van Genderen PJ, Rosenthal BM, Cramer JP, Visser LG, Muñoz J, Drew CP, Goldsmith CS, Steiner F, Wagner N, Grobusch MP, Plier DA, Tappe D, Sotir MJ, Brown C, Brunette GW, Fayer R, von Sonnenburg F, Neumayr A, Kozarsky PE, Tioman Island Sarcocystosis Investigation Team. Acute muscular sarcocystosis: an international investigation among ill travelers returning from Tioman Island, Malaysia, 2011-2012. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Nov 15:59(10):1401-10. doi: 10.1093/cid/ciu622. Epub 2014 Aug 4
[PubMed PMID: 25091309]
[25]
Lindsay DS, Dubey JP. Determination of the activity of pyrimethamine, trimethoprim, sulfonamides, and combinations of pyrimethamine and sulfonamides against Sarcocystis neurona in cell cultures. Veterinary parasitology. 1999 Apr 12:82(3):205-10
[PubMed PMID: 10348099]
[26]
Gottstein B. [Cyst-forming Coccidia: Toxoplasma, Neospora, Sarcocystis]. Schweizerische medizinische Wochenschrift. 1995 May 6:125(18):890-8
[PubMed PMID: 7770750]
[27]
Pathmanathan R, Kan SP. Three cases of human Sarcocystis infection with a review of human muscular sarcocystosis in Malaysia. Tropical and geographical medicine. 1992 Jan:44(1-2):102-8
[PubMed PMID: 1496700]
Level 3 (low-level) evidence
[28]
Dubey JP, Saville WJ, Sreekumar C, Shen SK, Lindsay OS, Pena HF, Vianna MC, Gennari SM, Reed SM. Effects of high temperature and disinfectants on the viability of Sarcocystis neurona sporocysts. The Journal of parasitology. 2002 Dec:88(6):1252-4
[PubMed PMID: 12537123]
[29]
Chhabra MB, Samantaray S. Sarcocystis and sarcocystosis in India: status and emerging perspectives. Journal of parasitic diseases : official organ of the Indian Society for Parasitology. 2013 Apr:37(1):1-10. doi: 10.1007/s12639-012-0135-y. Epub 2012 Aug 17
[PubMed PMID: 24431532]
Level 3 (low-level) evidence