Continuing Education Activity
Ovulation induction is the response of the ovary to exogenous stimulation by direct or indirect method. Ovulation induction is to alleviate infertility due to anovulation in females of reproductive age. This activity reviews the ovulation potential of a reproductive female and assisted ovulation in anovulatory females.
Objectives:
- Describe the physiology of ovulation and the appropriate tests for assessing ovarian reserve.
- Identify the indications and contraindications for ovulation induction and the potential complications of the medications prescribed for ovulation induction.
- Explain the clinical importance of lifestyle modification and medical and surgical methods of treatment in infertile women.
- Summarize the importance of coordination amongst the interprofessional team in providing positive pregnancy outcomes in infertile couples.
Introduction
The normal menstrual cycle and paracrine and endocrine mechanisms function through the hypothalamic-pituitary-ovarian axis (HPO axis). They are under neuroendocrinological control. The gonadotropin-releasing hormone (GnRH) is released in a pulsatile manner from the hypothalamus. Ovarian and uterine changes include follicular development and endometrial growth, respectively, which are essential for reproduction. In response to endogenous gonadotropin stimulation, there is rupture of the mature follicle, which releases secondary oocyte, known as ovulation. In the fallopian tubes, this secondary oocyte interacts with spermatozoa which leads to zygote formation. Implantation of this zygote into the uterus is called pregnancy.
Anovulation due to HPO axis dysfunction has been classified by WHO (world health organization) as under:[1]
- WHO class 1: Hypogonadotropic hypogonadism
- WHO class 2: Normogonadotropic hypogonadism
- WHO class 3: Hypergonadotropic hypogonadism
- WHO class 4: Hyperprolactinemia
Medications for ovulation induction have a major role in the production of the dominant follicles and endometrial growth. In addition, they prevent androgen to estrogen conversion, act as an antagonist on estrogen receptors; help in insulin sensitization of tissues; and direct stimulation of the hypothalamus through gonadotropins. Laparoscopic ovarian drilling is an adjunct second-line treatment.
These medical and surgical treatments aim to improve the chances of conception in otherwise infertile couples. In addition, this review highlights the role of ovarian reserve assessment, ovulation induction, and various techniques necessary for women having infertility due to anovulation.
Anatomy and Physiology
There are 2 ovaries, each 2 to 3 cm in size, and they lie on the posterior pelvic wall, lateral to the uterus. They are supported by suspensory ligaments, the tuboovarian ligament, and the broad ligament. Along with the production of oocytes, ovaries also produce sex steroid hormones (estrogen and progesterone) in response to pituitary gonadotrophins (LH and FSH).
Oogenesis
Oogenesis is the formation of oogonium. Oogenesis begins in intrauterine life, and oogonia are converted into the primary oocyte. The primary oocyte then undergoes meiosis-I and forms the secondary oocyte and a first polar body. Meiosis-I gets arrested in the diplotene stage of prophase at puberty. The outer part where primary oocyte and follicles are present is called the cortex, and the inner part is called the medulla. It contains the blood vessels and stromal cells (known as theca cells). Follicular cells present in the cortex are known as granulosa cells. These granulosa cells surround the primary oocyte.
These theca cells also surround the primary oocyte. The primary oocyte, along with surrounding granulosa cells and theca cells, is known as the primordial follicle. The maximum number of follicles are present at the fifth month of intrauterine life. At birth, there are about 1 to 2 million follicles. At puberty, follicles decrease to 4 to 5 lakh. Follicles undergo apoptosis even before the HPO axis is functional.
HPO axis becomes functional only at puberty. Therefore, the initial recruitment of follicles is hormone-independent.
Role of FSH
At puberty, the HPO axis of a female becomes functional. GnRH acts on the anterior pituitary, and FSH is released. FSH prevents follicles from undergoing atresia and apoptosis. FSH stimulates follicle growth.
FSH acts on granulosa cells of follicles, which in turn release estrogen and inhibin B. Estrogen has three main functions:
- Negative feedback on FSH
- Positive feedback on LH
- The proliferation of the endometrium of the uterus.
Estrogen decreases the level of FSH; thus, all follicles stimulated by FSH undergo apoptosis under the influence of estrogen except one, which is called the dominant follicle. It is a follicle that has the maximum number of FSH receptors. Therefore, estrogen has positive feedback on the levels of LH. This sudden increase in the level of LH hormone due to estrogen is known as LH surge. Estrogen levels must reach the level of 200 picograms and remain for at least 48 hours for LH surge to occur. Due to LH surge, meiosis-I, which was arrested till now, gets resumed. Therefore, Meiosis-I is hormone-dependent. The conversion of the primary oocyte to secondary oocyte is known as ovulation. The remaining cells of the follicle get converted into the corpus luteum.
Role of LH
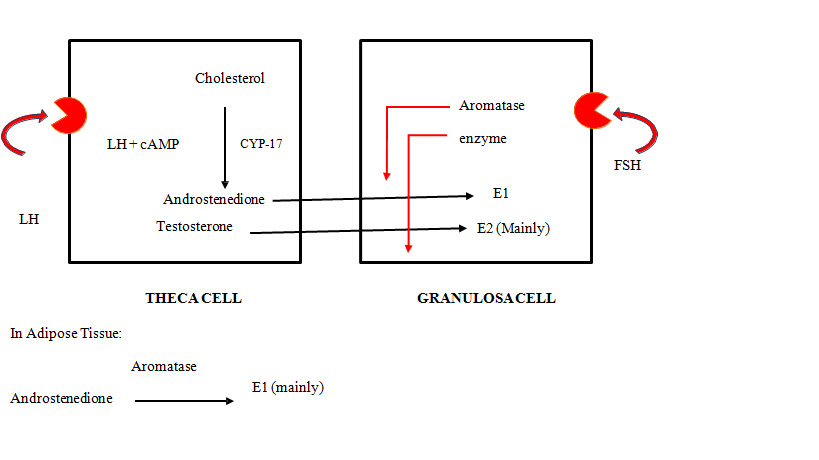
Receptors for LH hormone are present on the theca cells. LH acts on theca cells to produce androgens. These androgens are converted into estrogen under the effect of the aromatase enzyme. This conversion occurs in granulosa cells and adipose tissues as well. At the time of LH surge, LH acts on granulosa cells, causing luteinization of cells and release of progesterone in small quantities even before ovulation. Progesterone appears early in the menstrual cycle (at LH surge). In low concentrations, progesterone has a positive feedback effect on LH and FSH levels. Thus, levels of LH and FSH rise before ovulation. Ovarian steroidogenesis is explained by two cell two gonadotropin theory:[2]
|
Theca Cell
|
Granulosa Cell
|
|
Aromatase enzyme absent
|
Aromatase enzyme present
|
|
CYP 17 present
|
CYP 17 absent
|
|
Receptors for LH are present
|
Receptors for FSH are present
|
Follicular/ Proliferative Phase of the Menstrual Cycle
The ovarian follicular cycle is initiated by the FSH hormone. FSH acts on the granulosa cells to release estrogen. Estrogen decreases the level of FSH by negative feedback. Estrogen increases the level of LH by positive feedback. Estrogen causes the proliferation of the endometrium. Ovulation occurs on day 14, after the LH surge. Due to the low level of FSH under the negative feedback of estrogen, all the follicles undergo atresia except one, which is called the dominant follicle. This mature follicle continues to grow in size till it reaches the size of 18 to 20 mm for ovulation to occur. LH surge begins around 32 to 36 hours before ovulation; however, the peak of LH surge is around 10 to 12 hours before ovulation. LH surge is initiated by estrogen.
The time interval from the peak of estrogen to the peak of LH hormone is around 14 to 24 hours, and from estrogen peak to ovulation is 24 to 36 hours. LH surge is maintained by both estrogen and progesterone. LH and FSH surges occur before ovulation; however, ovulation occurs only due to LH surge. Meiosis-I, which was arrested, is resumed due to LH surge. This phase of the female cycle is the follicular phase because major changes occur in the follicle. The main hormone of this phase is estrogen, which leads to the proliferation of the endometrium. Therefore this phase is also known as the proliferative phase.
Ovulation
Some granulosa cells also surround the primary oocyte, which is now known as cumulus oophorus. LH comes in antral fluid during mid-cycle after LH surge. If LH is present in the antral cavity from early in the cycle, it will lead to atresia of the follicle. Normally, LH surge causes ovulation, i.e., conversion of the primary oocyte to the secondary oocyte. The follicle left after the ovulation is called the corpus luteum. During the intrauterine life of a female, there are 7 million oogonia that reduce to only 1 million at birth.[3] Pre LH surge perifollicular resistance index (RI) between 0.4 and 0.48 and peak systolic volume (PSV) of 10cm/sec are the indicators of mature follicle on ultrasound.[4]
Luteal/Secretory Phase of the Menstrual Cycle
Under the effect of LH, the corpus luteum starts growing, reaching its maximum size and maximum activity after 8 days of ovulation (Day 22 of the cycle). Corpus luteum releases progesterone mainly and also estrogen and Inhibin A. Progesterone has a negative feedback on LH. Progesterone supports the endometrium. At low concentration, progesterone increases LH and decreases FSH, but at high concentration, progesterone decreases LH levels and increases FSH levels. Due to an increase in progesterone, levels of LH decreases, and the corpus luteum also degenerate.
As a result, hormones secreted by the corpus luteum along with Inhibin A decreases. When the level of progesterone decreases, endometrial shedding occurs. This shedding of the endometrium is called menstruation. Progesterone is a smooth muscle relaxant, and a decrease in its levels causes a release of PGF2, which causes vasoconstriction. PGF2 alpha is also responsible for pain during menstruation, known as dysmenorrhoea. This phase is of the female cycle is known as the luteal phase, and the main hormone of this phase of the cycle is progesterone.
Indications
The following are the indications of ovulation induction:
- WHO Group II patients having anovulatory cycles
- Luteal phase defect
- Unexplained infertility
- Polycystic ovarian syndrome
- Controlled ovarian hyperstimulation in assisted reproductive technology (ART) cycles
- Superovulation in intrauterine insemination (IUI)
- Hyperprolactinemia
- Poor responders
Contraindications
Ovulation induction is contraindicated in:
- Hypergonadotropic hypogonadism
- Liver diseases
- Functional ovarian cysts (> 5cm)
- Tubal factors (tubal blockage/damage)
- Male factor infertility
- Severe grade 3 and 4 endometriosis
- Premature ovarian failure
- Genetic abnormalities (example: Turner syndrome)
Equipment
The requirements for successful ovulation induction are:
- Thermometer
- Microscope
- Urinary luteinizing hormone kit
- Ultrasound Doppler machine
- Clinical laboratory for hormonal assessment
- Drugs for ovulation induction
- Laparoscopy instruments
- Operation room
Personnel
An appropriate team is comprised of the following:
- Gynecologist and laparoscopic surgeon team
- Anaesthesia team
- Operation room nursing staff
- Lab assistants
- Radiologist
- Outpatient department nursing staff
- Pharmacist
- Counselor
Preparation
Prior to the ovulation induction, it is essential to assess the ovulation reserve.
Assessing ovulation reserve
Ovulation reserve is the capacity of the ovaries to produce fertilizable ova. This depends on the quality and quantity of ovarian antral follicles. Antral follicle count is considered to be a reliable parameter for ovarian reserve assessment.[5] Ovulation reserve tests can be divided as:
|
Clinical markers
|
Endocrinological markers
|
Ultrasound markers
|
|
Age
|
Day two or three serum FSH
|
Ovarian volume
|
|
Menstrual pattern
|
Day two or three serum oestradiol
|
Antral follicle count
|
|
|
Day three serum FSH or LH
|
Ovarian blood flow
|
|
|
Inhibin B
|
|
|
|
AMH
|
|
Technique or Treatment
Various techniques for ovulation induction are:
- Lifestyle modification for obesity-related infertility
- Medical methods
- Surgical methods
Lifestyle Modification for Obesity-related Infertility
Obesity is defined as a Body mass index (BMI) of more than 30 kg/m^2, and overweight is defined as BMI ≥25 kg/m^2.[6] Obesity has adverse effects on ovarian function and fertility. Obesity is associated with fewer chances of conception by both natural conception and ovulation induction resulting in conception. Patients with high BMI require a higher dose of ovulation-inducing drugs. High BMI also correlates with higher rates of miscarriage. Lifestyle modification methods like a healthy diet and exercise are helpful in the induction of ovulation in infertility due to anovulatory causes.[7] Lifestyle modification causes loss of weight, improves insulin resistance, and helps restore reproductive function. Dietary carbohydrates are important determinating factors in anovulatory infertility.[8] Between 5% to 10% loss of weight is sufficient to reinstate ovarian function. Lifestyle modification should be advised as the first line of treatment for infertility due to anovulation in obese patients.
Medical Methods
Anti-estrogens
Clomiphene Citrate (CC)
It is an anti-estrogenic drug. It binds with estrogen receptors at the hypothalamus, blocking the binding of estrogen hormone to estrogen receptors. Hypothalamus detects a lack of estrogen binding at the receptors and releases gonadotropin-releasing hormone (GRH). GRH signals the pituitary to secrete more follicle-stimulating hormone (FSH) and luteinizing hormone (LH).
FSH and LH stimulate the development of ovarian follicular. Estrogen level rises after follicular development; however, it does not decrease the levels of FSH and LH because there is no negative feedback on the hypothalamus as estrogen receptors are occupied by clomiphene citrate. Clomiphene citrate is a mixture of two isomers; enclomiphene (62%) and zuclomiphene (38%). Enclomiphene has a shorter duration of action and remains in the patient’s body for few days, while zuclomiphene remains in circulation for over a month.
Indications for clomiphene citrate include WHO type II group of patients (normal hypothalamus-pituitary ovarian axis); anovulation; luteal phase defect; unexplained infertility; endometriosis stage 1 and endometriosis stage 2. Prerequisites for prescribing clomiphene citrate are normal thyroid function test, normal prolactin function test, normal male partner evaluation, normal tubal patency, and normal adrenal functions. Contraindications include suspected pregnancy, hypergonadotropic hypogonadism, hypogonadotropic hypogonadism, liver diseases, large functional ovarian cysts, and a history of hypersensitivity to any drug. Adverse effects of clomiphene citrate include vasomotor flushes, mood swings, nausea, blurred vision, visual disturbances, breast tenderness, pelvic discomfort, ovarian cancer, and OHSS (ovarian hyperstimulation syndrome). The starting dose of clomiphene citrate is 50 to 100 mg, and it can be started from day 2 through 5 of the menstrual cycle. The maximum dose should not exceed more than 150 mg. It should not be given for more than 6 ovulatory cycles.
Tamoxifen
Tamoxifen is useful in inducing ovulation in patients with failed clomiphene citrate treatment cycles. Rates of spontaneous abortions are lower in women having conception with tamoxifen or letrozole compared with clomiphene citrate.[9]
Aromatase Inhibitor
Letrozole
Letrozole is an aromatase inhibitor and is used for ovulation induction. The main advantage of Letrozole is that it is a reversible enzyme inhibitor. It blocks the conversion of androgen to estrogen and thus increases the level of testosterone. It is used in a dose of 2.5 to 5 mg orally from day 2 or 3 of the cycle for five days. Its half-life is about 30 to 60 hours. Because of this short half-life of 4 to 5 hours, it is an ideal ovulation-inducing agent. Indication for its use is in clomiphene citrate resistant cases, PCOS patients, poor responders, endometriosis, an older woman, and breast cancer patients. Its major advantage over clomiphene citrate is that it has no anti-estrogenic effect on the endometrium and cervical mucus. It reduces multiple pregnancy rates and decreases the chance of OHSS. The use of letrozole has been recently increased in anovulatory infertility in comparison to clomiphene citrate.[10][9] The major adverse effects are vasomotor symptoms such as hot flashes, nausea, and fatigue.[11] It is contraindicated in women with the risk of osteoporosis, endometrial hyperplasia, and endometrial neoplasia.
Dopamine Agonists
Cabergoline is used as a dopamine agonist. It reduces vascular permeability. It decreases receptor expression for vascular endothelial growth factors. Hence, cabergoline reduces the incidence of OHSS.[12] It should be started on the day of the trigger when human chorionic gonadotropin (hCG) is given at a dose of 0.5 mg daily for 8 days. No significant risk is seen with administration for 8 days at a low dose. The most common side effects are headache, nausea, and dizziness.
Insulin Sensitizers
Metformin is used for ovulation induction. Metformin increases pregnancy rates in PCOS patients.[13] It causes minor gastrointestinal related disturbances. It is given 500 mg daily once a day and increased by 500 mg twice a day after 2 weeks.
Gonadotropins
These directly stimulate the ovaries, which stimulates the hypothalamus and indirectly increases the FSH and LH simultaneously. Bruno Lunnenfeld first reported the use of human menopausal gonadotropin (hMG). The various FSH hormone preparations available are hMG, hMGhp, urinary FSH, FSHhp, recombinant FSH and recombinant FSH, and recombinant LH combination. Indications of gonadotropin therapy are hypogonadotropic hypogonadism, hypothalamus-pituitary dysfunction, CC resistant patients, CC failure patients, superovulation combined with IUI controlled ovarian hyperstimulation in ART cycles.
Surgical Methods
Laparoscopic ovarian drilling was first introduced by Gjonnaess in 1984. This procedure is less invasive when compared with wedge resection and involves producing multiple holes on the ovary and surface using either electrocautery or laser. Electrosurgical reduction in the volume of ovarian stroma decreases ovarian androgen production and provided a better follicular environment. Reduction in androgen production causes lesser peripheral aromatization, elevated FSH levels, and re-establishing the HPO axis.
Complications
- Ovarian hyperstimulation syndrome
- Multiple pregnancies
- Spontaneous pregnancy loss
- Congenital anomalies
- Breast and ovarian cancer
Clinical Significance
Ovulation induction is the simplest and minimally invasive technique used in patients with anovulatory infertility. The method of ovulation induction selected by the clinician is based upon the underlying cause of anovulation and efficacy, cost, risks, and potential complications associated with each method. Women with ovulatory disorders must undergo conventional ovulation induction techniques before considering assisted reproductive techniques, as the success rates are good. In addition, if monitored by an experienced clinician, complication rates are low.
Induction of ovulation should be differentiated from stimulation of multiple follicle development in ovulatory women (controlled ovarian hyperstimulation) as done with assisted conception techniques. The main aim of this treatment modality is inducing monofollicular rather than multi follicular development and subsequent ovulation resulting in pregnancy and ultimately the birth of a healthy newborn, maximizing the rates of singleton pregnancies and minimizing the risk of OHSS (ovarian hyperstimulation syndrome).
Enhancing Healthcare Team Outcomes
Ovulation induction techniques are considered crucial in treating anovulatory infertility amongst infertile couples. It is considered that diagnosing the type of anovulation, assessing the ovarian reserve, assessing the male and female investigations, strategizing the medications and protocols is the key to treat couples with infertility. Physical and emotional support is also essential. Lifestyle modifications would also improve the fertility rates in patients. These will help enhance the infertility specialist's ability to initiate follow-up studies and decide the treatment protocol. Collaboration with radiologists, anesthesiologists, and nursing care teams is imperative for providing better outcomes. Trained and competent nurses are an integral part of the health care interprofessional team.
To achieve better outcomes, the treatment strategies have to be streamlined. Therefore, the need for meticulous planning and discussion with other professionals involved in treating the female is recommended to improve the fertility outcomes in infertile couples.
Nursing, Allied Health, and Interprofessional Team Interventions
The ovulation induction technique requires an interprofessional team of infertility specialists, gynecologists, advanced gynecological ultrasonography specialists, anesthesiologists or nurse anesthetists, nurses, pharmacists, and many other staff members, which should be in constant communication to provide the absolute best care to the patient. The multidisciplinary approach plays a vital role in the management and potential outcome of the patient.
Nursing care is essential as they educate the women undergoing IVF regarding the importance of ovulation induction. They help the women to take medications with the correct route, dose, and timing of medicine. This improves the outcome of pregnancy drastically—interprofessional team management and proper communication help provide better outcomes for patients undergoing ovulation induction.
Nursing, Allied Health, and Interprofessional Team Monitoring
The role of the infertility specialist, radiologist, anesthesiologist, and overall nursing team is continually expanding to increase the fertility rates among the couples undergoing ovulation induction. In addition, nursing staff provides their help in the initial consultation, assistance during transvaginal ultrasound scanning, and administration of medications at a proper time along with the appropriate dosage.
Interprofessional team monitoring is critical for the success of the ovulation induction and improving the overall patient outcome. Every team member is responsible for monitoring for any complications that may arise during the entire treatment process.