Continuing Education Activity
Persistent corneal epithelial defects result from failure of re-epithelialization and complete healing within two weeks of corneal epithelial insult, even after conventional treatment. Though not commonly encountered, they pose a management challenge as they are often refractory to standard supportive treatment. Persistent epithelial defects necessitate prompt and vigilant treatment to avoid associated potential complications, such as infection, vision loss, corneal scarring, melting, and even perforation. This activity reviews the etiopathogenesis, clinical features, diagnosis, current and novel management options for persistent epithelial defects. It highlights the role of the interprofessional team in the care of affected patients.
Objectives:
- Describe the pathophysiology of the persistent epithelial defect.
- Identify the risk factors for developing a persistent epithelial defect.
- Summarize the appropriate evaluation of persistent epithelial defect.
- Review the management options for the persistent epithelial defect.
Introduction
The corneal epithelium provides a smooth optical surface and maintains a protective barrier against infectious agents and damage, serving as the first line of ocular immune defense. Any breach in the continuity of the epithelium renders the eye susceptible to infection, ulcers, and even perforation.[1][2] Most of the corneal abrasions re-epithelialize rapidly and uneventfully; however, they persist for longer than usual duration in the presence of certain risk factors. Beyond approximately two weeks of standard treatment, they are described as persistent epithelial defects (PEDs).[2][3]
Though uncommon, their management is quite challenging since they are refractory to standard therapy and require a long-term follow-up. Without appropriate treatment, they can have potentially blinding consequences like scarring, infection, vascularization, and vision loss.[4] This article encompasses the etiopathogenesis, predisposing factors, diagnosis, current management options, and novel therapies under pipeline.
Etiology
The various etiological factors that can disrupt the normal corneal epithelial healing process can be broadly classified into the following categories.[5]
Defective Epithelial Adhesion
Proposed Mechanisms
- Defective adherence of epithelial cells to the basement membrane
- Deficient or abnormal basement membrane
- Matrix metalloproteinases (MMPs) overproduction
- Disruption of migration of regenerating epithelial cells
Associated conditions - Recurrent corneal erosions, epithelial basement membrane dystrophies, band-shaped keratopathy, bullous keratopathy, Salzmann nodular degeneration
Limbal Stem Cell Deficiency
- Proposed mechanism- Deficient regeneration of disrupted epithelium
- Associated conditions- Limbal stem cell deficiency (LSCD), chemical injury, trauma
Inflammation
Proposed Mechanisms
- Overactivity of inflammatory markers
- Excess production of chemotactic factors, growth factors, proteases
- Proliferation and migration of regenerating epithelial cells
- Stromal remodeling and even melting
Associated conditions- Autoimmune diseases, keratoconjunctivitis sicca, rosacea, Sjogren syndrome, peripheral ulcerative keratitis, Mooren ulcer, Steven-Johnson syndrome, graft versus host disease
Neurotrophic
- Proposed mechanism- Disruption of corneal innervation
- Associated conditions- Diabetes mellitus, herpetic keratitis, anesthetic abuse, severe dry eye, post-traumatic nerve damage
Surface Trauma
- Proposed mechanism- Epithelial cell loss exceeds the regeneration capacity of epithelial stem cells due to recurrent mechanical insult to the ocular surface.
- Associated conditions- Entropion, ectropion, lagophthalmos, trachoma, severe dry eye, corneal injury due to chemical or thermal burns
Hereditary
- Proposed mechanisms- Hereditary deficient limbal stem cells, abnormal basement membrane
- Associated conditions- Aniridia, epithelial and basement membrane dystrophies
Epidemiology
The actual incidence of PED is not known. However, studies have estimated the total incidence of PED, based on the occurrence of underlying conditions per year, to be less than 200,000 cases in the U.S., identifying it as an orphan disease.[6] In addition, in the U.S., PED has been reported per year in 7558 patients undergoing corneal transplantation and in 2,480 to 5,257 cases of diabetic vitrectomy. Diabetic keratopathy, present in 47% to 64% of patients with diabetes, aggravates the risk of PED.[6]
Risk factors for post-operative epithelial defects in patients undergoing penetrating keratoplasty for infectious keratitis include male gender, > 60 years of age, graft size > 9 mm, bacterial or viral cause, associated comorbidities like rheumatic disease, and chemotherapy.[7] High incidence of PED has also been reported in patients who undergo pars plana vitrectomy and in children on mechanical ventilation, warranting precautions and immediate intervention.[8][9]
Pathophysiology
Disruption of the integrity of the corneal epithelium activates a highly regulated cascade over 7 to 14 days comprising proliferation and migration of epithelial cells, adherence to the underlying intact basement membrane, extracellular matrix remodeling, and eventual apoptosis or quiescence.[10][11]
The epithelium takes approximately seven days to develop, regenerate and adhere to the intact basement membrane. The stroma can adhere to this layer only following re-epithelialization, failing which tissue defects can develop. This entire process involves a complex interplay of various growth factors and cellular signals, namely epidermal growth factor (EGF), fibroblast growth factor (FGF), hepatocyte growth factor (HGF), keratocyte growth factor (KGF), platelet-derived growth factor (PDGF), tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), insulin-like growth factor-1 (IGF-1), transforming growth factor-beta (TGF-β).
Any variation in this highly regulated process can result in abnormally slow healing. For example, if the stroma is affected along with the basement membrane, the epithelium will take around eight weeks for complete reattachment, resulting in a PED. The defect extends into the stroma and involves the proliferation of myofibroblasts with the resultant disordered extracellular matrix, developing ulceration, stromal melting, and scarring.
History and Physical
Patients usually present with pain, watering, and foreign body sensation in the affected eye. However, these features may be absent in neurotrophic PEDs. There may be blurring of vision, redness, photophobia, pain with blinking, and eye movements. PED, if left untreated, can lead to corneal inflammation, infection, ulceration, scarring, melting, and even perforation due to disruption of the protective epithelial and stromal layers of the cornea.[4]
A comprehensive history comprising duration, mechanism of trauma, prior treatment received and surgeries, associated ocular and systemic comorbidities, family history, the immune status will help arrive at an etiological diagnosis and allow more specific treatment. Distinguishing an epithelial defect from a PED can be done based on the time required for complete healing after an injury. An epithelial defect recovers by 7 to 10 days, while a PED will not heal even after two weeks, and such a patient is usually refractory to standard treatments and supportive care.[5]
Evaluation
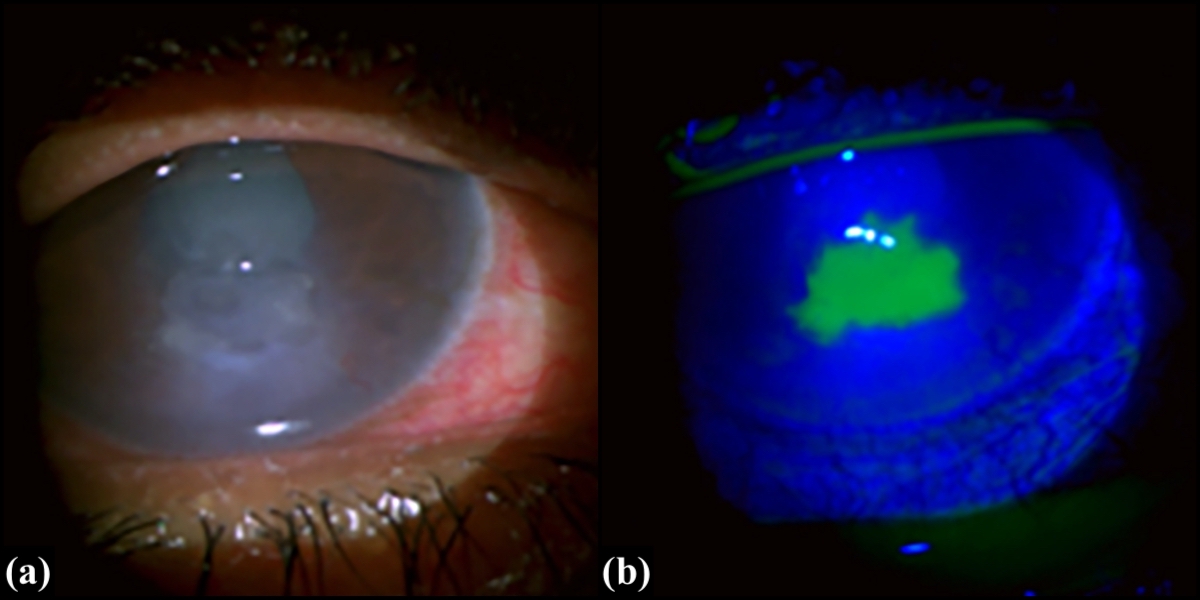
A thorough examination of both the eyes, adnexa, and systemic assessment is of utmost importance to determine the etiology. Fluorescein staining is used to photograph the defect for serial monitoring and measuring the size, depth, and location of the epithelial defect using a slit lamp.[12]
Deeper PEDs take longer to absorb the fluorescein into the stroma and frequently have grayish-white edges with heaped-up epithelium. Associated signs of infection, such as underlying haze and infiltrates and anterior chamber inflammation, must be actively sought. There can be associated basement membrane dystrophies, nodular degeneration, dendrites, limbal stem cell deficiency, conjunctival injection, features of superior limbic keratoconjunctivitis, allergic conjunctivitis, foreign body, and concretions. It is essential to assess dry eyes using the Schirmer test and tear-film breakup time. The Rose Bengal dye stains devitalized epithelial cells, implying impaired tear function, regardless of gross tear flow.
It is important to remember to examine the ocular adnexa. Syringing and regurgitation on pressure over the lacrimal sac (ROPLAS) are routinely performed to rule out any local source of infection. The punctum position, lid globe apposition, lid abnormalities, trichiasis, blepharitis, and meibomitis must be considered. Periorbital and facial asymmetry should be carefully evaluated to rule out facial nerve palsy. Finally, one must assess corneal sensations to exclude neurotrophic factors.
The diagnosis of PED is primarily clinical. It can be supplemented by investigations that help to quantify the lesion and track its progress. AS-OCT is a non-invasive definitive technique for quantifying epithelial thickness and loss over time, especially in areas of corneal thinning and scarring.[13] Confocal microscopy is a technique that allows the imaging of thin layers of living corneal cellular structures in real-time. In vivo HRT-II confocal microscopy has been designed for studying the superficial and peripheral corneal and conjunctival epithelium.[14]
For systemic comorbidities, relevant systemic investigations must be conducted. For example, in patients with diabetes, fasting and postprandial blood sugar levels and HbA1C values are measured. Vitamin A deficiency can be confirmed by measuring serum retinol-binding protein and zinc levels. Rheumatoid factor, anti-cyclic citrullinated peptide (anti-CCP) antibody for rheumatoid arthritis, antinuclear antibody (ANA), anti-double-stranded DNA (anti-dsDNA) antibody for systemic lupus erythematosus (SLE), anti-Ro/SSA, and anti-La/SSB antibody for Sjogren syndrome are all autoimmune indicators.
Treatment / Management
The primary goal of the therapy is to create an environment conducive to the migration and proliferation of regenerating epithelial cells. Multiple factors influence the treatment regimen, including concurrent condition, overall systemic status, prior medication use, and response to therapy. If an underlying infiltrate is noticed, aggressive measures to address the infectious element must be taken. The priority is to control the infectious process from progressing to corneal melt or perforation.[15] Furthermore, certain topical drugs are known to be toxic to the cornea and can impede epithelial healing.[16][17] Gentamicin and tobramycin, for example, are topical aminoglycosides that can cause superficial punctate keratitis and delay corneal re-epithelialization. More importantly, benzalkonium chloride, a widely used preservative, is a recognized ocular surface irritant with toxic effects. Healthcare professionals should alter a patient's medication regimen to reduce the effect of medicamentosa and provide more favorable conditions for corneal healing. Frequent and multiple follow-ups with a thorough examination at every visit are mandatory to ensure the efficacy of the treatment.
A step-by-step approach is used to manage PEDs, beginning with conservative treatment and progressing to more aggressive options if refractory. The first and most crucial step is to determine the potential etiological factor. Next, the underlying condition must be targeted for local treatment to be effective, such as nerve growth factor for neurotrophic keratopathy, metabolic control in diabetic keratopathy, medication withdrawal in iatrogenic causes, and limbal stem cell transplant for limbal stem cell deficiency.
The standard medical treatment protocol begins with aggressive lubrication using preservative-free artificial tears and sterile ocular ointments.[1] They provide a setting in which the epithelium can reestablish standard structure and function. It is critical to discontinue all concurrent medications that may disrupt or delay re-epithelialization. Many commercial artificial tears contain preservatives, most commonly benzalkonium chloride, to prevent contamination with bacteria and fungi. These preservatives are known to irritate the eyes and, if used frequently, can cause ocular toxicity and epithelial damage.[18] As a result, it is advisable to use preservative-free lubricants for better outcomes.
The next step in the treatment of PED is the insertion of punctal plugs for temporary or permanent occlusion. They increase the retention of lubricants, which supplements normal corneal healing. The retention allows greater availability of growth factors such as EGF, TGF-β, FGF, HGF, fibronectin, calcitonin gene-related peptide, vitamin A and C. Any toxic ocular medication must be stopped before using punctal plugs. Commercially available are silicone and collagen plugs.[1][19]
Bandage soft contact lenses (BCL) help protect the damaged epithelium from erosion by eyelid blinking, thus aiding re-epithelialization. Secondary infection should be avoided using topical broad-spectrum antibiotics in conjunction with preservative-free artificial tears, particularly neurotrophic keratitis.[20] Pressure patching can be used as an alternative to protect the cornea during re-epithelialization. However, it is necessary to monitor it every 1 to 2 days because it can impair healing and be a source of infection. Temporary cyanoacrylate glue can help with pressure patching by preventing stromal melting and acting as a bacteriostat.[21]
Oral tetracyclines are beneficial due to their anti-collagenase activity, which inhibits matrix metalloproteinases from inflammatory mediators and promotes healing. The use of topical corticosteroids is debatable due to the increased risk of infection, tissue destruction, and stromal melting.[22] They are effective in PED caused by herpetic keratitis, Steven-Johnson syndrome, atopic keratoconjunctivitis, Sjogren syndrome, and graft versus host disease.[2]
In cases where medical management has failed, surgical interventions such as debridement and tarsorrhaphy can be beneficial.[1] Debridement removes inactive, stagnant epithelium from the edges of the PED, allowing new regenerating epithelial cells to migrate. Temporary tarsorrhaphy is advantageous because it reduces the evaporation of tears by decreasing the area of the exposed cornea while also promoting easy access to the cornea for drug delivery.[23] Nonsurgical eyelid closure can be achieved by injecting Botulinum toxin A into the levator palpebrae superioris muscle.[24]
Treatment of Refractory Cases
Patients who do not respond to the modalities discussed above are labeled as refractory cases. Amniotic membrane grafting or transplant (AMT) is effective in refractory cases by providing a scaffold for re-epithelialization, anti-inflammatory properties, and decreasing vascularization and scarring.[1][25] It contains numerous growth factors, proteinase inhibitors, and proteins that aid in healing. It can be fibrin-glued or sutured beneath a BCL or inserted as a self-retaining device. Post-operative infection, suture breakage, membrane dissolution, and hemorrhage are among the few reported complications.
One potential treatment is the use of autologous serum eye drops (ASE), prepared by centrifuging the patient's serum and diluting it in concentrations of 20%, 50%, and even 100%. Its role in PED healing is attributed to several serum constituents that contribute to multiple steps in the healing process, such as vitamin A, E, EGF, PDGF, TGF-β, nerve growth factor, and insulin-like growth factor. Patients with systemic illness, poor general health, active infection, or diseases with high levels of pro-inflammatory cytokines in the serum are not good candidates for ASE. In such cases, alternative whole-blood derivatives such as umbilical cord blood serum (CBS) tears and platelet-rich fibrin (PRF) tears can be used. Despite promising results, whole-blood-derived products pose a significant challenge due to transportation risks and high supply costs.[26]
A new scleral contact lens, also known as the Prosthetic Replacement of the Ocular Surface Ecosystem (PROSE), has shown promising results.[27] It has protective effects on the epithelium, as well as high oxygen permeability and lubricating properties. Its radial venting channels keep the cornea moist and provide an extra route for oxygen delivery even during sleep. It also eliminates friction between the eyelid and the healing corneal epithelium. Microbial keratitis is a potential risk, and prophylactic antibiotics are recommended.[28] Limbal stem cell transplantation is beneficial in underlying stem cell deficiency, such as Steven-Johnson syndrome, ocular cicatricial pemphigoid, and extensive chemical injury.[29] Phototherapeutic keratectomy can help by using a laser to create strong adhesions to the basement membrane and Bowman layer.[30] Boston keratoprosthesis has emerged as a potential option in cases of multiple failed corneal transplants.[31]
Novel Therapies
Thymosinβ4 is an amino-acid peptide that inhibits the synthesis of transcription factors mediating inflammation.[32] It has been found to promote PED healing through increased keratocyte migration, collagen deposition, and angiogenesis. A new product is available that is a novel selective down regulator of connexin 43, a cellular gap junction protein.[33] It has shown encouraging results in the recovery of PED by inhibiting the intercellular exchange of apoptotic factors and signaling molecules that cause lesion spread and increased defect size. Corneal neurotization is a new surgical technique for treating neurotrophic keratopathy that helps restore corneal nerve sensations by using a peripheral nerve graft such as the contralateral supraorbital or supratrochlear nerves.[34]
Topical fibronectin may be beneficial because it is known to promote cellular adhesions in wound healing. However, conflicting reports about its benefits in PED have limited its clinical use.[3][6] The interaction of various growth factors governs the corneal epithelial healing process. Therapies that act on the molecular level to enhance or inhibit these factors have been studied. These include topical epidermal growth factor (EGF), insulin-like growth factor-1 (IGF-1), human growth hormone, albumin eye drops, amniotic membrane extract eye drops, matrix regenerating agent, and recombinant human nerve growth factor (rhNGF), also known as cenegermin-bkbj ophthalmic solution.[35][36][37][38][39]
Differential Diagnosis
Making a diagnosis of an epithelial defect is not difficult; however, identifying it as a persistent epithelial defect is more important for early intervention and prevention of destructive sequelae. An epithelial defect heals entirely in 7 to 10 days, whereas a persistent epithelial defect will not heal in even two weeks. The focus should be on identifying the underlying etiology to provide targeted treatment.[7]
Prognosis
Despite a number of available treatment options, the outcome of PED in recovery remains variable. Few defects may respond quickly with a single intervention, whereas others may take weeks to resolve, necessitating a multi-modal approach. The key to a good prognosis is early diagnosis and treatment because defects treated early recover more rapidly and completely. Therefore, the primary objective is to intervene as soon as possible to allow for rapid re-epithelialization and minimize secondary complications.
Complications
- Infection
- Ulceration
- Stromal scarring
- Melting
- Perforation
Deterrence and Patient Education
PED management necessitates vigilant monitoring with frequent follow-up visits even daily until resolution. Patients should be educated on the importance of adherence to treatment and follow-up. Since effective drug delivery is crucial for treatment efficacy, the appropriate technique for administering drugs should be demonstrated. Following complete resolution, clinicians should explain to the patient all prophylactic measures to prevent a recurrence.
Pearls and Other Issues
PEDs, while uncommon, can have disastrous consequences if not treated. Avoiding these requires timely, stringent management, good adherence to therapy, and close monitoring. They are frequently resistant to standard treatment regimens causing the disease to progress and necessitating more aggressive treatment with frequent follow-up visits. A step-by-step management algorithm and newer interventions can help with re-epithelialization in such patients. Given that PEDs treated earlier heal faster, it may be prudent to consider prophylactic treatment and a more aggressive approach at the outset in certain at-risk eyes.
Enhancing Healthcare Team Outcomes
The success of PED is dependent on several factors, including the identification of the underlying etiology, a stepwise treatment approach, adherence to treatment, follow-up, and prevention of recurrence. This requires a collaborative endeavor of an interprofessional team of specialists. It is always important to rule out an infectious etiology, where the role of a microbiologist is undeniable.
Patients with underlying autoimmune problems should be referred to a rheumatologist for a comprehensive systemic evaluation before commencing immunomodulators and titrating dosages based on clinical response. In the manufacture, storage, and dispensing of autologous serum, which is an essential component of the treatment regimen, the role of a compounding pharmacist is fundamental. The nurses are pivotal for patient care because they ensure that treatment protocols are followed and that the patient is educated. Active participation of every team member, combined with closed-loop communication, will aid in achieving desired outcomes and minimizing morbidity.