Continuing Education Activity
Explore the forefront of ophthalmic diagnostics with this course on orbital color Doppler imaging (CDI). This innovative modality provides a comprehensive approach to assessing orbital blood flow, proving indispensable in the diagnosis, prognostication, and serial follow-up of various ophthalmic diseases. CDI evaluates 5 parameters, including Doppler indices, central retinal artery, ophthalmic artery, short posterior ciliary arteries, and superior ophthalmic vein, enabling assessment of various ocular conditions such as intrabulbar tumors, arterial occlusions, glaucoma, and diabetic retinopathy.
Participating clinicians learn the procedural steps of orbital CDI and understand its application in clinical settings. Additionally, clinicians learn the crucial role of the interprofessional team in effectively evaluating patients undergoing sequential orbital CDI, fostering collaborative approaches to enhance patient care and outcomes.
Objectives:
Identify the indications for orbital color Doppler imaging, such as evaluating orbital masses, assessing orbital vascular disorders, and monitoring intraocular pressure.
Differentiate between normal and abnormal orbital blood flow patterns on color Doppler imaging.
Apply orbital anatomy and physiology knowledge to interpret color Doppler imaging findings in the context of specific orbital pathologies.
Implement the applications of orbital color Doppler imaging in ocular disorders managed by an interprofessional team.
Introduction
Color Doppler imaging (CDI) is a non-invasive technique for two-dimensional visualization of normal and pathological vasculature. CDI of the orbital vessels has been used for diagnosis, prognostication, and serial follow-up of ocular pathologies.[1] CDI is an effective tool for researching and understanding the pathogenesis of various ophthalmic diseases. As an imaging modality, CDI visualizes blood supply to the orbital, comprising the internal and external carotid artery circulation. Orbital blood flow is regulated by the autonomic nervous system and chemical mediators, depending on the hemodynamic status. As such, CDI may be utilized during diagnosis, prognosis, and follow-up, with some indications still under research.
CDI has 5 evaluation parameters: Doppler indices, central retinal artery, ophthalmic artery, short posterior ciliary arteries, and superior ophthalmic vein. Based on these measurements, several ocular conditions are evaluated, including but not limited to persistent fetal vasculature, intrabulbar tumors, arterial occlusions, glaucoma, diabetic retinopathy, and vascular malformations.
Anatomy and Physiology
The arteries and veins of the orbit show a great degree of interindividual variation.[2] The orbit is supplied primarily by the internal carotid artery circulation with a minor contribution from the external carotid artery circulation.
The principal artery of the orbit is the ophthalmic artery (OA). OA is the first intracranial branch of the ICA after it leaves the cavernous sinus. The OA enters the orbit through the optic canal, inferolateral to the optic nerve. The OA runs inferior to the optic nerve, gives rise to the central retinal artery, and then crosses to the superomedial aspect of the optic nerve. The medial and lateral long posterior ciliary arteries and multiple short posterior ciliary arteries supply the outer coats of the globe. The OA passes between the medial rectus and the superior oblique muscles, gives multiple muscular branches, supraorbital artery, anterior and posterior ethmoidal arteries, and terminates near the superomedial orbital margin to form the supratrochlear and the dorsal nasal arteries. The external carotid artery contributes to the infraorbital artery and an orbital branch from the middle meningeal artery to the orbit.
The orbital venous system comprises 2 main veins, the superior and the inferior ophthalmic veins, and multiple variable veins. The superior ophthalmic vein drains into the cavernous sinus, passing through the superior orbital fissure. The inferior ophthalmic vein can be a tributary of the superior ophthalmic vein or drain directly into the cavernous sinus.
The orbital blood flow is autoregulated by the activity of the autonomic nervous system and chemical mediators, as listed below.
- Endothelin-1: A potent vasoconstrictor affecting choroidal blood flow and primarily a risk factor for glaucoma and diabetic retinopathy.
- Nitric Oxide: A vasodilator molecule regulating the intraocular pressure and orbital blood flow during orbital inflammation.
- Adenosine: A potent molecule modulating the IOP that causes retinal vasodilation.
- Oxygen: The partial pressure of oxygen in retinal vessels inversely correlates with the blood flow, affecting the retinal autoregulation mechanisms.
- Carbon Dioxide: Carbon dioxide partial pressure directly controls the retinal blood flow since it affects retinal autoregulation more strongly than oxygen.
- Other molecules like estrogen and carbonic anhydrase enzyme have an unclear role in autoregulation.
Exercise and sympathetic stimulation cause arteriolar vasoconstriction and decrease the ocular blood flow.[3][4]
Indications
The following are indications for orbital color Doppler imaging:
Diagnostic Adjunctive Role
- Persistent fetal vasculature
- Intrabulbar tumors
- Arterial occlusion
- Ocular ischemic syndrome
- Carotid cavernous fistula
Prognostication and Follow-up
- Glaucoma
- Thyroid eye disease
- Diabetic retinopathy
- Vascular malformations
- Mucormycosis
Indications Still Under Research
- Age-related macular degeneration
- Central retinal vein occlusion
- Central serous chorioretinopathy
- Sickle cell disease
- Behcet disease
- Orbital cysticercosis
Contraindications
The absolute contraindication for orbital ultrasonography and CDI is a history of penetrating or perforating trauma to the globe, presenting with globe rupture.
Equipment
The ultrasonography machine offering duplex mode: Two-dimensional real-time ultrasonography with color-coded Doppler imaging is required. The orbital vessels are imaged at a depth of 1.5 to 4.0 cm. A linear probe with a frequency of 7.5 to 10 Mhz is preferred.[5]
Personnel
An expert radiologist or a well-trained ophthalmologist should perform the CDI.
Preparation
An ultrasound console with a 10 MHz linear probe, ultrasound jelly, and a transparent plastic film is needed for a CDI. The patient should avoid smoking or alcohol consumption at least 12 hours before the study. Applying topical drugs, especially anti-glaucoma medications, influences the orbital blood flow. Factors like hypertension, anxiety, tachycardia, high cardiac output, and increased pulse pressure alter the retrobulbar hemodynamics.[5] The examiner should try to minimize the effects of the abovementioned factors on the CDI study.
Technique or Treatment
The patient is asked to lie down with the head elevated by 30º and look straight ahead with eyelids shut gently. The ultrasound probe is placed horizontally over the eyelid to get an axial view of the orbit. The probe should exert minimal pressure over the globe to avoid compression of orbital vessels.
Doppler Indices
- Peak systolic velocity: The maximum velocity recorded during the systole phase of the Doppler waveform.
- End diastolic velocity: The minimum velocity recorded during the diastole phase of the Doppler waveform.
- Resistivity index: The ratio of the difference between peak systolic velocity and end-diastolic velocity to the value of the peak systolic velocity during the same cycle of the Doppler waveform.[6]
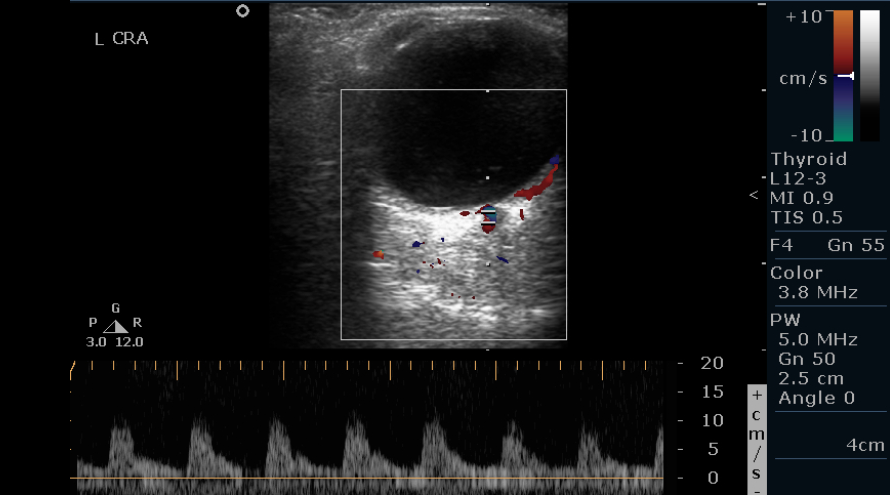
- Imaging of the central retinal artery: The central retinal artery (CRA) is localized in the optic nerve head at 1 to 10 mm behind the posterior wall of the globe. The CRA has an estimated diameter of 0.2 mm. CRA shows a low resistance flow low peak systolic velocity, and a rapid flow in the diastolic phase. CRA requires the placement of a small Doppler gate for reliable measurements. The central retinal vein is a small-caliber vessel located close to the CRA. The central retinal vein has a flow waveform below the zero lines (see Image. Normal Central Retinal Artery Doppler of the Left Eye).
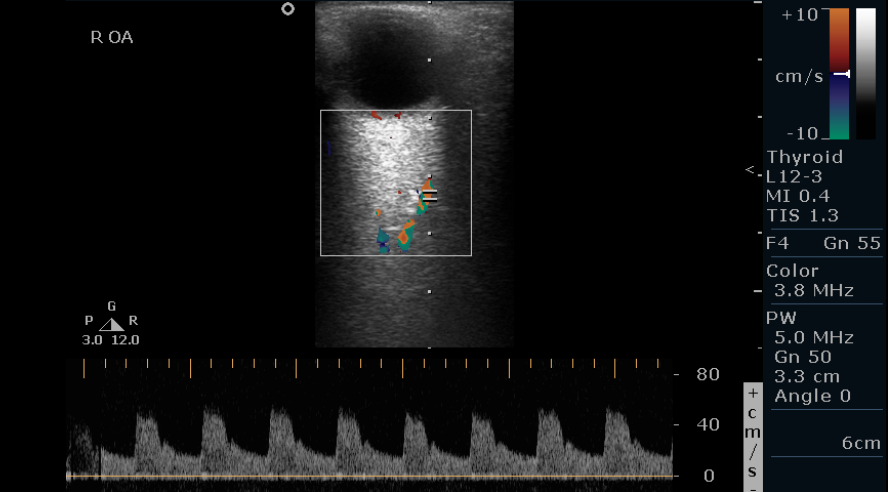
Imaging of the OA: The OA is imaged in the nasal orbit as it crosses the ON at a depth of 35 to 36 mm. The diameter is usually 0.7 to 1.5 mm. The OA is imaged, making an angle less than 60° between the ultrasound beam and vessel lumen. It has a moderate to high resistance flow, a rapid systolic peak with a dichotomous notch, and a sharply decreasing diastolic phase. (see Image. Normal Ophthalmic Artery Doppler of the Right Eye).
Imaging of the short posterior ciliary artery vessels: The short posterior ciliary artery vessels appear as numerous branches lateral and medial to the ON at 1 to 3 mm behind the posterior pole. The vessels have a diameter of 0.1 to 0.2 mm. Their flow is a low-resistance waveform with velocities between the OA and the CRA flow velocities. These vessels also require the placement of a small doppler gate. The values are often variable on subsequent measurements due to variations in the beam angle during each successive measurement.
Imaging of the SOV: The SOV is localized in the superomedial orbit. Due to the absence of valves and flaccidity of the orbital veins, the venous flow waveform depends on multiple external factors.
The flow velocities vary depending on the depth of Doppler imaging, with a deeper vascular localization yielding a falsely low flow waveform. Definite reporting is done after averaging the results of at least 3 Doppler waveforms and flow-velocity values on repeat imaging. Vast differences in the values of these indices have been seen between populations. The average values observed have been compiled below (see Table. Average Doppler Waveforms and Flow-Velocity Values).
Table. Average Doppler Waveforms and Flow-Velocity Values
|
Doppler Index
(Mean + standard deviation)
|
Kaiser et al [7]
(Study location: Switzerland)
|
Fukukita et al [8]
(Study location: Japan)
|
Rusia et al [9]
(Meta-analysis)
|
Goel et al [10]
(Study location: India)
|
| OA PSV (cm/s) |
39.6 + 5.87 |
35.8 + 8.4 |
36.84 + 7.38 |
33.06 + 4.03 |
| OA EDV (cm/s) |
11.02 + 1.71 |
8.03 + 2.40 |
9.30 + 2.91 |
7.27 + 2.50 |
| OA RI |
0.76 + 0.05 |
0.776 + 0.035 |
0.74 + 0.06 |
0.77 + 0.05 |
| CRA PSV (cm/s) |
11.02 + 1.71 |
11.3 + 3.1 |
12.01 + 3.26 |
12.71 + 3.30 |
| CRA EDV (cm/s) |
3.23 + 0.86 |
4.18 + 1.06 |
3.46 + 1.21 |
2.33 + 0.87 |
| CRA RI |
0.71 + 0.05 |
0.627 + 0.038 |
0.7 + 0.08 |
0.77 + 0.05 |
| SPCA PSV (cm/s) |
11.43 + 1.92 |
10.2 + 2.8 |
11.93 + 2.52 |
NA |
| SPCA EDV (cm/s) |
3.74 + 1.06 |
3.95 + 1.27 |
3.94 + 1.07 |
NA |
| SPCA RI |
0.68 + 0.06 |
0.614 + 0.06 |
0.66 + 0.1 |
NA |
OA, ocular artery; PSV, peak systolic velocity; EDV, end-diastolic velocity; CRA, central retinal artery; RI, resistivity index; SPCA, short posterior ciliary artery.
Complications
Orbital CDI may result in complications like pain due to excessive pressure on the globe and globe damage in previously undiagnosed traumatic globe rupture cases. Minimal complications have been reported in the hands of trained personnel.
Clinical Significance
Persistent Fetal Vasculature
Based on their ultrasonography and CDI characteristics, persistent fetal vasculature (PFV) cases can be classified into 4 types:
- Type I: I-shape
- Type II: Y-shape
- Type III: Inverted Y-shape
- Type IV: X-shape
Sonographic screening and CDI of PFV cases allow visualization of the vascularised membranes and definitive surgical planning. The pars plana incision and surgery can be performed in types I and III, whereas types II and IV require an anterior surgical approach under direct visualization.[11]
Intrabulbar Tumors
The vascularity and resistivity index of the orbital vessels differentiate between malignant and benign lesions. Choroidal melanoma shows a higher peak systolic velocity and resistivity index in the feeder vessels than orbital cavernous hemangiomas. The response to radiation therapy is monitored by CDI parameters like reduction in the vascularised areas, decrease in peak systolic velocity, and elevation of tumor echogenicity.[12] Magnetic resonance imaging-CDI fusion imaging is an upcoming modality that may enable improved diagnostics and follow-up.[13]
Arterial Occlusions
The central retinal artery occlusion (CRAO) or the OA occlusion (OAO) presents as sudden onset painless vision loss. High resistivity index and absent flow in the CRA, with a high resistivity index and normal flow velocities in the OA and the short posterior ciliary artery, suggest CRAO. Acute OAO usually presents as a hemodynamically empty orbit, with collateral circulation opening up in the chronic OAO orbits.[14]
Ocular Ischemic Syndrome
Misdiagnosis of ocular ischemic syndrome (OIS) in clinics is common, which leads to missing the critical bailout period. OIS often presents with an absent or reverse end-diastolic flow in the OA and the CRA.[15] This "steal phenomenon" is specific for an underlying high-grade ICA occlusion or stenosis.[16] CDI aids in early diagnosis, which is potentially life-saving.
Arteritic Anterior Ischemic Optic Neuropathy
Giant cell arteritis (GCA) patients with no evident ocular involvement show a marked decrease in blood flow in bilateral orbits. The absence of flow in the long posterior ciliary artery, very high flow in the OA, and altered flow in the CRA are common findings with GCA.[17] This is an important predictor of an impending arteritic anterior ischemic optic neuropathy (A-AION) and warrants prompt management. The established A-AION cases present findings similar to CRAO.
Carotid Cavernous Fistula
Evaluation of the SOV in cases of post-traumatic proptosis may show a reverse flow, suggesting a flow from the orbital apex towards the globe. The waveform analysis may show an arterialized high-velocity blood flow through a low-resistance blood vessel, signifying a high-flow fistula between the intracavernous part of the ICA and the cavernous sinus. The SOV resumes normal venous flow in rare cases, showing spontaneous resolution of carotid cavernous fistula (CCF).[18] The gold standard for evaluating and planning the embolization of CCF is carotid angiography.
Glaucoma
The pathogenesis of open-angle glaucoma is a failure of autoregulation of the OBF with poor intraocular pressure (IOP) control.[19] Orbital CDI shows a characteristic decrease in the flow velocities and is high in progressive glaucoma patients. Lower peak systolic velocity in the OA, the CRA, the short posterior ciliary artery, and resistivity index values higher than 0.75 in the OA signify ongoing glaucomatous damage to the ON.[20] The CDI parameters improve with adequate IOP control on anti-glaucoma medications and post-filtration surgeries.[21] Monitoring for hemodynamic changes in CDI when the visual fields and the IOP are within the normal range can lead to an early diagnosis and correct treatment.
Thyroid Eye Disease
The active thyroid eye disease (TED) orbits show inflammatory changes and higher flow velocities. The inactive TED orbits may develop external compression of the vasculature, causing a high resistivity index and decreased flow velocities. CRA and OA flow patterns differ significantly between healthy and TED orbits. The OA-peak systolic velocity and OA-EDV can be used to differentiate the severity of TED.[10] Dysthyroid optic neuropathy can occur with low peak systolic velocity in the CRA, causing ON ischemia.[5] Immediate intervention for DON cases by immunomodulation or orbital surgical decompression reduces the resistivity index and improves the arterial flow velocities. SOV flow pattern also correlates with orbital apex crowding in muscle-predominant inactive TED orbits.[22] SOV flow direction on CDI can predict dysthyroid optic neuropathy in such cases.
Diabetic Retinopathy (DR)
Diabetes mellitus is characterized by neuropathy and failure of the normal autoregulation of OBF. The ischemic retina stimulates the release of vascular endothelial growth factor (VEGF) and alters the flow velocities before the onset of frank changes.[23] Treatment of proliferative diabetic retinopathy with pan-retinal photocoagulation, intravitreal steroids, or intravitreal anti-VEGF injections shows a significant decrease in arterial flow velocities. Early diagnosis and strict metabolic control can help prevent severe visual loss with DR.[24]
Vascular Malformations - Infantile Periocular Hemangioma
The orbital vascular malformations show a characteristic high flow pattern on CDI. Managing periocular hemangiomas with oral propranolol reduces the intra-lesional blood flow and peak systolic velocity in the feeding vessels. The CDI findings on follow-up visits help predict the chances of complete remission.[25]
Mucormycosis
Orbital mucormycosis is an angioinvasive fungus affecting the CRA and OA. In CRA or OA occlusion cases, the respective artery shows an absent waveform on CDI. Reduced PSV and EDV, with raised resistivity index, can also help predict an impending occlusion. Since the CDI findings correlate with clinical, radiological, and pathological findings, Doppler imaging is a potential marker for prognostication in mucormycosis.[26]
Age-Related Macular Degeneration
Exudative age-related macular degeneration (ARMD) shows high resistivity index and decreased arterial blood flow. This suggests a retinal and choroidal vasculature involvement in the pathogenesis of ARMD.[27]
Central Retinal Vein Occlusion
Acute central retinal vein occlusion shows a rise in resistivity index of the central retinal artery and reduced peak systolic velocity in the CRA and the OA. No significant changes exist in the hemodynamics post-intra-vitreal anti-VEGF injections.[28]
Central Serous Chorioretinopathy
Choroidal hyperperfusion is proposed to be the leading cause of central serous chorioretinopathy (CSCR). In patients with unilateral CSCR, a decrease in resistivity index and arterial flow velocities in both eyes suggests a systemic etiology.[29]
Sickle Cell Disease
Sickle cell disease causes early microvascular occlusion, an increased resistivity index, and decreased flow velocity in the CRA. Subsequent retinal hypoxia causes vasodilation and increases the flow velocity in the OA.[30] These changes may help detect ocular involvement before the objective signs are visible.
Behcet Disease
Behcet disease presents with widespread vasculitis, including orbital circulation. Vascular occlusion in the retina and the choroid leads to a high resistivity index and reduced flow velocities in the central retinal artery and the short posterior ciliary artery.[31] Detecting changes in blood flow before clinical signs may improve early management and visual outcomes.
Orbital Cysticercosis
Orbital cysticercosis presents with a cystic lesion in the extra-ocular muscles. The active inflammation can cause an increase in vascularity in the muscle belly around the cyst.[32]
Enhancing Healthcare Team Outcomes
Orbital CDI is a rapid, non-invasive, and accessible modality for imaging of orbital vessels. Trained personnel can view the orbital vasculature changes and corroborate the diagnosis. Early management with a serial follow-up leads to positive visual outcomes. The ophthalmologist, the radiologist, and the nursing staff play an invaluable role in the efficient functioning of the team.
Nursing, Allied Health, and Interprofessional Team Monitoring
Minimal monitoring is required in the post-imaging period.