Introduction
The mucous membrane that lines the structures within the oral cavity limits is known as oral mucosa. This is a wet soft tissue membrane that extends from the junction between the vermilion border of the lips and labial mucosa anteriorly to the palatopharyngeal folds posteriorly.
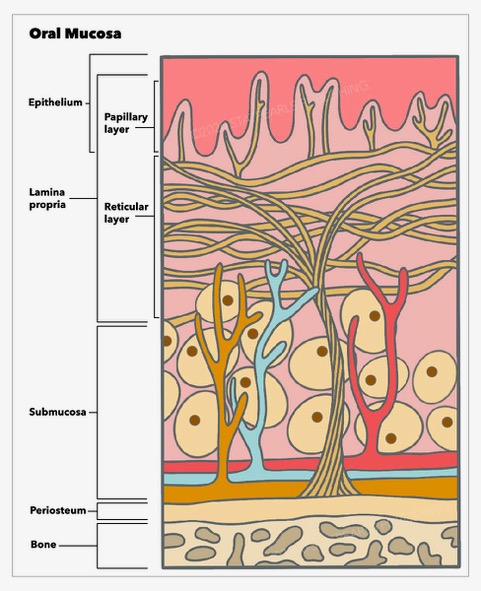
Histologically, the oral mucosa is formed by three layers, a surface squamous stratified epithelium, known as the oral epithelium, whose thickness and degree of keratinization depend on the location and functional requirements [1]. Beneath is an underlying connective tissue, known as lamina propria, and a dense irregular connective tissue, the submucosa, which is found at the deepest level. The latter is absent in some oral cavity regions, where the lamina propria is directly bound to the bone or muscle.
The oral mucosa can be classified into lining mucosa, masticatory mucosa, and specialized mucosa, with distinctive histological, clinical, and functional features. The variety of functions that the oral mucosa develops mainly includes protecting the underlying tissues from mechanical, chemical, and biological stimuli, secretion of essential substances, and a sensory function, which allows the perception of temperature, touch, pain, and taste [2][3][4][2].
Structure
Oral Epithelium
The entire surface of the oral mucosa is covered by squamous stratified epithelium. This is a highly organized, avascular, and semipermeable tissue whose thickness and degree of keratinization varies according to the location in the oral cavity and the area's functional and mechanical requirements. An interdigitated interface connects the epithelium and the lamina propria. The undulating projections of the deeper layer of the epithelium, known as rete pegs, attach to the underlying papillary projections of the lamina propria. Between these two tissues, there is a non-cellular basement membrane to which the epithelium is tightly bound. The basement membrane provides support to the epithelium and connects them to the connective tissue. On light microscopy, it is seen as a line of demarcation between the epithelium and connective tissue of the lamina propria. However, it is better observed with electron microscopy that clearly shows the basal lamina, which is further subdivided into lamina lucida and lamina densa.
The oral mucosa can be classified into three types that differ histologically, clinically, and functionally. The mucosa that lines the mobile structures of the mouth receives the name of lining, or moveable mucosa, which is found on the soft palate, cheeks, lips, alveolar mucosa, the floor of the mouth, and vestibular fornix. The type of epithelium that covers the lining mucosa is a non-keratinized stratified squamous epithelium [1]. The rigid mucosa tightly bound to the underlying bone in the attached gingiva and hard palate is known as masticatory mucosa. The type of epithelium covering these surfaces is a keratinized or para-keratinized stratified squamous epithelium, which provides the masticatory mucosa its capacity to better support the stress upon which it is subjected during mastication. Finally, there is a specialized mucosa on the dorsum of the tongue that shows a squamous stratified epithelium that can be either keratinized or non-keratinized. It receives this name because of its unique feature of having different types of lingual papillae and taste buds that allow taste perception. Because the dorsum of the tongue actively participates in mastication, this mucosa is sometimes also classified as masticatory mucosa.
Four layers form the oral epithelium in keratinized oral mucosa, which is the case of the masticatory mucosa. Commencing from the deeper layer, we found the stratum basale followed by stratum spinosum, stratum granulosum, and stratum corneum. When the epithelium is nonkeratinized, such as in the lining mucosa, above the stratum basale, there is a stratum filamentosum and stratum distendum [1]. Moreover, the non-keratinized epithelium, seen on the lining mucosa, lacks the granular layer, and the spinous layer is known to be generally thinner [5]. The cells that form the epithelium are attached to each other by desmosomes and get gradually flatten from the stratum basale upwards to the stratum corneum, where they acquired a scaly or squamous appearance. Squamous epithelial cells are known as keratinocytes since they are mostly composed of cytokeratins.
The stratum basale features a layer of cuboidal or columnar cells that are above the basement membrane to which hemidesmosomes attach them. These cells are known for their mitotic capacity. Just above the stratum basale, several layers of larger cells called prickle cells, due to their shape, form the stratum spinosum. The stratum granulosum comes; next, these cells contain small cytoplasmatic keratohyalin granules that strongly stain with hematoxylin. Finally, the more superficial layer, the stratum superficiale or stratum corneum, is a keratinized layer composed of very flat cells depicted by the lack of nucleus and by staining pink with eosin [6].
Oral Epithelium Replenishment
The oral epithelial cells are frequently replaced by cell division, around each 14 to 21 days. This is because the oral cavity is constantly exposed to high functional demands, which necessitate frequent turnover. The replenishment process starts at the stratum basale, mainly formed by mitotic cells that undergo proliferation first and then a differentiation and migration process [2]. The turnover is known to be faster in the movable mucosa than the masticatory one (see table 1). To maintain the homeostasis of the epithelium, there is a need for differentiation and desquamation at the surface that balances cell division. When the homeostasis gets altered by factors such as aging or pathological conditions, it may result in a hyperplastic or atrophic epithelium [7].
Table 1: Turnover time of epithelial cells in selected tissues[7]
| Oral tissue |
Average epithelial turnover time (days) |
| Buccal mucosa |
14 |
| Floor of the mouth |
20 |
| Hard palate |
24 |
Non-keratinocyte Cell Population
Besides keratinocytes, other specialized cells permanently reside within the oral epithelium, referred to as non-keratinocyte cells, including melanocytes, Langerhan cells, and Merkel cells. Moreover, other various inflammatory cells may transiently migrate to the oral epithelium.
Melanocytes
Melanocytes are melanin-producing, elongated dendritic cells located in the basal layer of the oral epithelium that originate from the neural crest and then migrate to the skin and oral mucosa, where they reside [8]. These cells contain the proteins needed to synthesize melanin and for the maturation process of melanosomes. Melanocytes synthesize melanin as structures known as melanosomes that are then transferred into the cytoplasm of adjacent epithelial cells thanks to their long dendritic projections that extend between the keratinocytes. The ratio of melanocytes to keratinocytes in the oral epithelium's stratum basale ranges from 1:10 to 1:15 [9][10][11]. Melanocyte stem cells, whose niche in the oral cavity is still unknown, maintain the population of mature melanocytes thanks to their capacity for regeneration and differentiation [10].
All individuals have the same number of melanocytes in the oral mucosa and skin. The different skin colors and pigmentation of the oral mucosa are determined by the size and quantity of the melanosomes and the type of melanin synthesized, eumelanin or pheomelanin. Melanin granules, which are groups of melanosomes, can be microscopically observed in a heavily pigmented tissue stained with hematoxylin and eosin.
The function that the melanocytes perform in the human body, although not completely understood, is known to be the production of melanin that contributes to the determination of the color of the skin, mucosa, hair, and eyes. At the same time, melanin protects these tissues from the detrimental effect of ultraviolet light, reactive oxygen species, and free radicals present in the environment [10]. It is worth noting that with age, the number of oral melanocytes rises, and therefore there is an increased extent and intensity of oral pigmentations that are considered physiological [12][13][14]. It is suspected that this augmentation may result from cumulative possible melanogenic stimuli such as inflammatory conditions, medications, recurrent and mild functional injuries, or tobacco smoke [15].
Langerhans Cells
Langerhans cells are dendritic cells derived from the bone marrow that migrate to the oral epithelium, where they reside within the stratum spinosum. They are essential in the tissue's immune surveillance since they function as antigen-presenting cells by phagocytosing antigens in the epithelium and migrating to the underlying lamina propria, from where they can reach the regional lymph nodes. Here they transform the antigen proteins into antigenic peptides, which are consequently presented to T cells [16]. Therefore, Langerhans cells are the link between the oral mucosa and the immune system. An ultrastructural distinctive feature of these cells is the rod-shaped organelles, sometimes described as "tennis rackets," found exclusively in the cytoplasm of Langerhans cells, called Birbeck granules or bodies. Langerhan cells can be observed with specific immunohistochemical reactions, such as S-100 immunohistochemistry.
Merkel Cells
Merkel cells are slowly adapting sensory touch receptors associated with a neural sensitive ending located primarily in the epidermis; however, they are also found in the oral mucosa within the stratum basale [17][18]. They have cytoplasmatic vesicles usually located next to the nerve fibers linked to them. It has been suggested that these structures release transmitter molecules into the synapse-like junction located between the nerve fiber and the Merkel cell, which generates the nervous impulse.
In the oral cavity, they are mostly located in the keratinized epithelium of the maxillary and mandibular gingivae and hard palate. However, these Merkel cell-neurite complexes are located in higher numbers in the mucosa of the lingual gingivae. Therefore, it is believed that they act as slow adapting mechanoreceptors and give somatosensory information on the tongue's position [18].
Patients who wear complete dentures are known to decrease oral perceptibility due to the loss of periodontal ligament. Recently studies have raised the possibility that an increase in Merkel cells may help to partially compensate the loss of mechanoreception from the loss of periodontal ligament in such patients. Despite this, edentulous patients’ oral perceptibility will remain compromised [19].
Lamina Propria
Underneath the epithelium, there is a layer of connective tissue termed lamina propria composed of blood vessels, nerves, fibroblasts, macrophages, mast cells, and inflammatory cells fibers all immersed in an amorphous substance formed by proteoglycans and glycoproteins. The lamina propria is subdivided into two layers: the superficial papillary layer and the deeper reticular layer. The papillary layer is formed by thin collagen fibers irregularly oriented, forming undulating papillae ridges that connect with the epithelium; this surface provides a wider area for nutrient transport [20]. In the papillary layer, many capillary loops are found. The reticular layer is located between the papillary layer and the underlying structure (submucosa or periosteum according to the region) and is formed by thicker collagen fibers that orient parallel to the surface, although the basal fibers gradually arrange to perpendicularly connect to the periosteum [21]. These fibrous attachments are called mucoperiosteum, which provides the capacity to resist compression and shear to the oral mucosa due to a firm connection to the bone [22].
The principal cell found in the lamina propria is the fibroblast, which undertakes essential functions. It participates in the synthesis and replenishment of the connective fibers and the amorphous substance and takes part in wound healing, where the number of fibroblasts increases. In some conditions, such as drug-induced gingival overgrowth, the inducing drugs trigger the activation and proliferation of the gingival tissues' fibroblasts, resulting in an increased secretion of the glycosaminoglycan of the amorphous substance [23]. Macrophages mainly participate in phagocytic activities and also stimulate fibroblast proliferation during wound healing. Finally, mast cells are also found in the connective tissue of the lamina propria. Their distinctive feature is cytoplasmatic granules containing heparin and histamine, the latter being known for initiating vascular changes in the inflammatory process. The two main fibers found in the connective tissue of the lamina propria are collagen and elastin, where collagen fibers type I and III are the principal ones.
Submucosa
Underneath the lamina propria, there is a layer of fibrocollagenous and elastic tissue containing blood vessels and nerves known as submucosa. According to the location, the submucosa may contain adipose tissue, minor salivary glands, lymphoid tissue, and muscle. The submucosa is found in all the buccal cavity regions except the attached gingiva and the hard palate covered by masticatory mucosa, where the submucosa layer is absent, and the lamina propria is directly attached to the underlying bone, forming a mucoperiosteum.
Ectopic sebaceous glands, known as Fordyce granules, may be found on the submucosa layer of the oral mucosa in some locations. Even though they have always been considered a normal variation, recent studies have suggested that people with increased lipid profiles have a higher number of Fordyce granules. Hence this clinical finding should not be disregarded. They are mostly found on the buccal mucosa and labial mucosa. Age and smoking reduce their density [24].
Function
Protective Function
The oral cavity is an environment constantly challenged by the mechanical, chemical, and biological stimuli of our daily activities. The oral mucosa plays an essential role in protecting the underlying tissues from mechanical forces involved in the normal function of mastication (stretching, compression, and abrasion from a hard diet), external antigens, and noxious molecules from the diet. Furthermore, the oral mucosa is also exposed to carcinogenic substances found in alcohol, tobacco, and betel nut, consumed in some regions. The oral epithelium acts as a barrier against these physiological and pathogenic stresses. It functions as a physical and an immune barrier to external aggressions and prevents the penetration of the oral cavity's normal bacterial flora that may cause infection. The oral epithelium achieves this by being composed of multilayers of epithelial cells and cell-cell junctions and maintaining immune responses to antigens thanks to the presence of dendritic cells (DCs) and T helper 17 cells (Th17) [2].
Secretion
The main substance secreted by the oral mucosa is saliva, which is released by the ducts of the major and minor salivary glands. The minor salivary glands widely distributed in the oral cavity are included in the submucosa. Still, the major salivary glands, which are the main source of saliva, are located outside the limits of the oral mucosa. However, their excretory ducts open into the oral cavity contributing to the maintenance of the wetness of the tissue.The oral mucosa possesses a less significant amount of sebaceous glands reported on the lips, labial, and buccal mucosa in the majority of the adult population and may be found sporadically in the alveolar mucosa. These glands secret a fatty substance, known as sebum, whose functions have not yet been determined. However, recent studies of skin sebum suggest that it may play a role in immunity [25].
Sensory Function
The oral cavity receives its sensory innervation from the three branches of the trigeminal nerve. Three types of sensory endings are mainly present in the oral mucosa, consisting of Merkel’s disks, Meissner’s corpuscles, and free nerve endings, which allow the oral mucosa to perceive and respond to the stimulus of temperature, touch, and pain. Furthermore, it perceives the taste sensations of salty, sweet, sour, bitter, and umami, although it has been recently suggested that there may also be a taste sensation of fat [3][4]. The taste receptor cells are located orally in the dorsum of the tongue and the soft palate. The larynx, pharynx, and upper esophagus mucosas also contain these receptors.The sensory function of the oral cavity is essential for the identification of objects, influencing the actions performed during mastication, and initiating the swallowing reflex. The sense of touch permits the coordination of movements required by the tongue, lips, and soft palate to correctly emit sounds when speaking [26].
Clinical Significance
The oral mucosa has a large number of functions of clinical significance. It acts as a protective physical and immune barrier from external stimulus and harbors minor salivary glands that secret saliva maintaining the wetness of the tissue, and perceives and responds to the stimulus of temperature, touch, and pain. Multiple diseases may affect the oral mucosa and impair its capacity to develop these tasks. To mention some examples, the oral epithelium disruption can cause various oral mucosal lesions such as oral lichen planus and oral leukoplakia. Oral carcinomas, such as squamous cell carcinoma (OSCC), have been developed when the oral epithelial barrier of the mucosa is disrupted and keratinocytes undergo defective differentiation [2]. Furthermore, the gingival barrier destruction caused by dysbiosis in the oral microbiota combined with inflammation leads to periodontal diseases [2].
Common superficial oral mucosa lesions include recurrent herpes labialis, candidiasis, recurrent aphthous stomatitis, erythema migrans, hairy tongue, and lichen planus [29]. Also, it is worth noting that abnormal oral mucosa may be a symptom of mucosal or skin diseases and many systemic conditions. Therefore, knowledge of the histological features of the oral mucosa is imperative to recognizing and accurately diagnosing pathological benign conditions and malignancies.