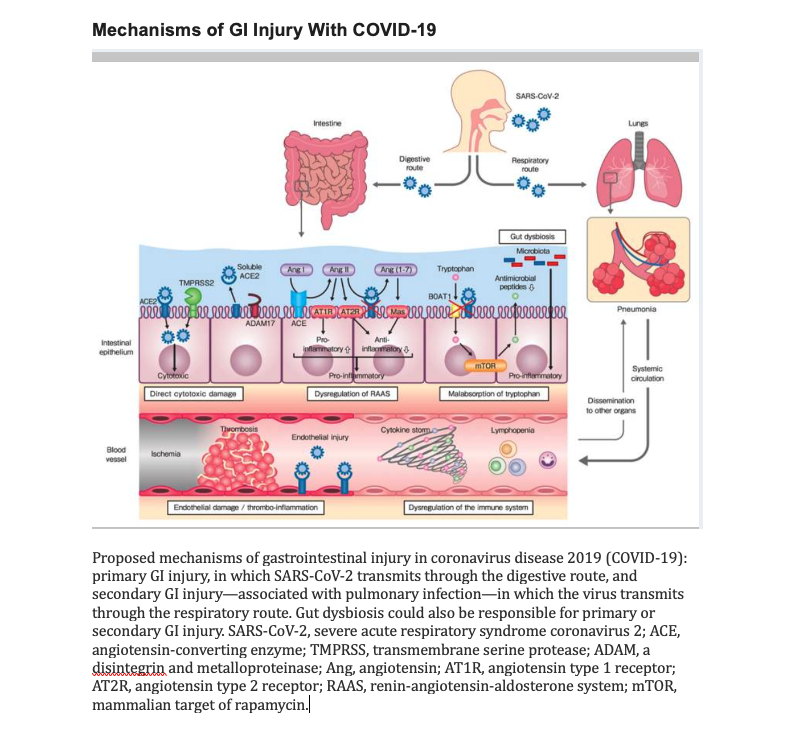
[1]
Pan L, Mu M, Yang P, Sun Y, Wang R, Yan J, Li P, Hu B, Wang J, Hu C, Jin Y, Niu X, Ping R, Du Y, Li T, Xu G, Hu Q, Tu L. Clinical Characteristics of COVID-19 Patients With Digestive Symptoms in Hubei, China: A Descriptive, Cross-Sectional, Multicenter Study. The American journal of gastroenterology. 2020 May:115(5):766-773. doi: 10.14309/ajg.0000000000000620. Epub
[PubMed PMID: 32287140]
Level 2 (mid-level) evidence
[2]
Cholankeril G, Podboy A, Aivaliotis VI, Pham EA, Spencer SP, Kim D, Ahmed A. Association of Digestive Symptoms and Hospitalization in Patients With SARS-CoV-2 Infection. The American journal of gastroenterology. 2020 Jul:115(7):1129-1132. doi: 10.14309/ajg.0000000000000712. Epub
[PubMed PMID: 32618665]
[3]
Mao R, Qiu Y, He JS, Tan JY, Li XH, Liang J, Shen J, Zhu LR, Chen Y, Iacucci M, Ng SC, Ghosh S, Chen MH. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: a systematic review and meta-analysis. The lancet. Gastroenterology & hepatology. 2020 Jul:5(7):667-678. doi: 10.1016/S2468-1253(20)30126-6. Epub 2020 May 12
[PubMed PMID: 32405603]
Level 2 (mid-level) evidence
[4]
Mitsuyama K, Tsuruta K, Takedatsu H, Yoshioka S, Morita M, Niwa M, Matsumoto S. Clinical Features and Pathogenic Mechanisms of Gastrointestinal Injury in COVID-19. Journal of clinical medicine. 2020 Nov 11:9(11):. doi: 10.3390/jcm9113630. Epub 2020 Nov 11
[PubMed PMID: 33187280]
[5]
Pegoraro F, Trapani S, Indolfi G. Gastrointestinal, hepatic and pancreatic manifestations of COVID-19 in children. Clinics and research in hepatology and gastroenterology. 2022 Apr:46(4):101818. doi: 10.1016/j.clinre.2021.101818. Epub 2021 Oct 2
[PubMed PMID: 34607068]
[6]
Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clinical immunology (Orlando, Fla.). 2020 Jun:215():108427. doi: 10.1016/j.clim.2020.108427. Epub 2020 Apr 20
[PubMed PMID: 32325252]
[7]
Chan JF, Kok KH, Zhu Z, Chu H, To KK, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging microbes & infections. 2020:9(1):221-236. doi: 10.1080/22221751.2020.1719902. Epub 2020 Jan 28
[PubMed PMID: 31987001]
[8]
Sze S, Pan D, Nevill CR, Gray LJ, Martin CA, Nazareth J, Minhas JS, Divall P, Khunti K, Abrams KR, Nellums LB, Pareek M. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine. 2020 Dec:29():100630. doi: 10.1016/j.eclinm.2020.100630. Epub 2020 Nov 12
[PubMed PMID: 33200120]
Level 2 (mid-level) evidence
[9]
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020 Apr 16:181(2):271-280.e8. doi: 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5
[PubMed PMID: 32142651]
[10]
Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020 May:158(6):1831-1833.e3. doi: 10.1053/j.gastro.2020.02.055. Epub 2020 Mar 3
[PubMed PMID: 32142773]
[11]
Zou X, Chen K, Zou J, Han P, Hao J, Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Frontiers of medicine. 2020 Apr:14(2):185-192. doi: 10.1007/s11684-020-0754-0. Epub 2020 Mar 12
[PubMed PMID: 32170560]
[12]
Lin L, Jiang X, Zhang Z, Huang S, Zhang Z, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020 Jun:69(6):997-1001. doi: 10.1136/gutjnl-2020-321013. Epub 2020 Apr 2
[PubMed PMID: 32241899]
Level 3 (low-level) evidence
[13]
Effenberger M, Grabherr F, Mayr L, Schwaerzler J, Nairz M, Seifert M, Hilbe R, Seiwald S, Scholl-Buergi S, Fritsche G, Bellmann-Weiler R, Weiss G, Müller T, Adolph TE, Tilg H. Faecal calprotectin indicates intestinal inflammation in COVID-19. Gut. 2020 Aug:69(8):1543-1544. doi: 10.1136/gutjnl-2020-321388. Epub 2020 Apr 20
[PubMed PMID: 32312790]
[14]
Ojetti V, Saviano A, Covino M, Acampora N, Troiani E, Franceschi F, GEMELLI AGAINST COVID‐19 group. COVID-19 and intestinal inflammation: Role of fecal calprotectin. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2020 Nov:52(11):1231-1233. doi: 10.1016/j.dld.2020.09.015. Epub 2020 Sep 22
[PubMed PMID: 33060042]
[15]
Vodnar DC, Mitrea L, Teleky BE, Szabo K, Călinoiu LF, Nemeş SA, Martău GA. Coronavirus Disease (COVID-19) Caused by (SARS-CoV-2) Infections: A Real Challenge for Human Gut Microbiota. Frontiers in cellular and infection microbiology. 2020:10():575559. doi: 10.3389/fcimb.2020.575559. Epub 2020 Dec 9
[PubMed PMID: 33363049]
[16]
de Nies L, Galata V, Martin-Gallausiaux C, Despotovic M, Busi SB, Snoeck CJ, Delacour L, Budagavi DP, Laczny CC, Habier J, Lupu PC, Halder R, Fritz JV, Marques T, Sandt E, O'Sullivan MP, Ghosh S, Satagopam V, CON-VINCE Consortium, Krüger R, Fagherazzi G, Ollert M, Hefeng FQ, May P, Wilmes P. Altered infective competence of the human gut microbiome in COVID-19. Microbiome. 2023 Mar 9:11(1):46. doi: 10.1186/s40168-023-01472-7. Epub 2023 Mar 9
[PubMed PMID: 36894986]
[17]
Hoffmann D. The role of the oral cavity in SARS-CoV-2- and other viral infections. Clinical oral investigations. 2023 Jun:27(Suppl 1):15-22. doi: 10.1007/s00784-023-05078-z. Epub 2023 Jun 13
[PubMed PMID: 37310513]
[18]
Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver international : official journal of the International Association for the Study of the Liver. 2020 May:40(5):998-1004. doi: 10.1111/liv.14435. Epub 2020 Mar 30
[PubMed PMID: 32170806]
[19]
Bloom PP, Meyerowitz EA, Reinus Z, Daidone M, Gustafson J, Kim AY, Schaefer E, Chung RT. Liver Biochemistries in Hospitalized Patients With COVID-19. Hepatology (Baltimore, Md.). 2021 Mar:73(3):890-900. doi: 10.1002/hep.31326. Epub 2020 Nov 4
[PubMed PMID: 32415860]
[20]
Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. The lancet. Gastroenterology & hepatology. 2020 May:5(5):428-430. doi: 10.1016/S2468-1253(20)30057-1. Epub 2020 Mar 4
[PubMed PMID: 32145190]
[21]
Li J, Fan JG. Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. Journal of clinical and translational hepatology. 2020 Mar 28:8(1):13-17. doi: 10.14218/JCTH.2020.00019. Epub 2020 Mar 30
[PubMed PMID: 32274341]
[22]
Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. Journal of hepatology. 2021 Mar:74(3):567-577. doi: 10.1016/j.jhep.2020.09.024. Epub 2020 Oct 6
[PubMed PMID: 33035628]
Level 2 (mid-level) evidence
[23]
Özdemir Ö, Arsoy HEM. Commentary on COVID-19-induced liver injury in various age and risk groups. World journal of virology. 2023 Jan 25:12(1):44-52. doi: 10.5501/wjv.v12.i1.44. Epub
[PubMed PMID: 36743662]
Level 3 (low-level) evidence
[24]
Liptak P, Nosakova L, Rosolanka R, Skladany L, Banovcin P. Acute-on-chronic liver failure in patients with severe acute respiratory syndrome coronavirus 2 infection. World journal of hepatology. 2023 Jan 27:15(1):41-51. doi: 10.4254/wjh.v15.i1.41. Epub
[PubMed PMID: 36744167]
[25]
McNabb-Baltar J, Jin DX, Grover AS, Redd WD, Zhou JC, Hathorn KE, McCarty TR, Bazarbashi AN, Shen L, Chan WW. Lipase Elevation in Patients With COVID-19. The American journal of gastroenterology. 2020 Aug:115(8):1286-1288. doi: 10.14309/ajg.0000000000000732. Epub
[PubMed PMID: 32496339]
[26]
Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, Overbergh L, Gysemans C, Colli ML, Marchetti P, Mathieu C, Eizirik DL, Sebastiani G, Dotta F. SARS-CoV-2 Receptor Angiotensin I-Converting Enzyme Type 2 (ACE2) Is Expressed in Human Pancreatic β-Cells and in the Human Pancreas Microvasculature. Frontiers in endocrinology. 2020:11():596898. doi: 10.3389/fendo.2020.596898. Epub 2020 Nov 13
[PubMed PMID: 33281748]
[27]
Lax SF, Skok K, Zechner P, Kessler HH, Kaufmann N, Koelblinger C, Vander K, Bargfrieder U, Trauner M. Pulmonary Arterial Thrombosis in COVID-19 With Fatal Outcome : Results From a Prospective, Single-Center, Clinicopathologic Case Series. Annals of internal medicine. 2020 Sep 1:173(5):350-361. doi: 10.7326/M20-2566. Epub 2020 May 14
[PubMed PMID: 32422076]
Level 2 (mid-level) evidence
[28]
Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet (London, England). 2020 May 2:395(10234):1417-1418. doi: 10.1016/S0140-6736(20)30937-5. Epub 2020 Apr 21
[PubMed PMID: 32325026]
[29]
Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. The Lancet. Respiratory medicine. 2020 Apr:8(4):420-422. doi: 10.1016/S2213-2600(20)30076-X. Epub 2020 Feb 18
[PubMed PMID: 32085846]
[30]
Dorrell RD, Dougherty MK, Barash EL, Lichtig AE, Clayton SB, Jensen ET. Gastrointestinal and hepatic manifestations of COVID-19: A systematic review and meta-analysis. JGH open : an open access journal of gastroenterology and hepatology. 2021 Jan:5(1):107-115. doi: 10.1002/jgh3.12456. Epub 2020 Nov 21
[PubMed PMID: 33363257]
Level 1 (high-level) evidence
[31]
Hajifathalian K, Krisko T, Mehta A, Kumar S, Schwartz R, Fortune B, Sharaiha RZ, WCM-GI research group∗. Gastrointestinal and Hepatic Manifestations of 2019 Novel Coronavirus Disease in a Large Cohort of Infected Patients From New York: Clinical Implications. Gastroenterology. 2020 Sep:159(3):1137-1140.e2. doi: 10.1053/j.gastro.2020.05.010. Epub 2020 May 8
[PubMed PMID: 32389667]
[32]
Carvalho A, Alqusairi R, Adams A, Paul M, Kothari N, Peters S, DeBenedet AT. SARS-CoV-2 Gastrointestinal Infection Causing Hemorrhagic Colitis: Implications for Detection and Transmission of COVID-19 Disease. The American journal of gastroenterology. 2020 Jun:115(6):942-946. doi: 10.14309/ajg.0000000000000667. Epub
[PubMed PMID: 32496741]
[33]
Parasa S, Desai M, Thoguluva Chandrasekar V, Patel HK, Kennedy KF, Roesch T, Spadaccini M, Colombo M, Gabbiadini R, Artifon ELA, Repici A, Sharma P. Prevalence of Gastrointestinal Symptoms and Fecal Viral Shedding in Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis. JAMA network open. 2020 Jun 1:3(6):e2011335. doi: 10.1001/jamanetworkopen.2020.11335. Epub 2020 Jun 1
[PubMed PMID: 32525549]
Level 1 (high-level) evidence
[34]
Fix OK, Hameed B, Fontana RJ, Kwok RM, McGuire BM, Mulligan DC, Pratt DS, Russo MW, Schilsky ML, Verna EC, Loomba R, Cohen DE, Bezerra JA, Reddy KR, Chung RT. Clinical Best Practice Advice for Hepatology and Liver Transplant Providers During the COVID-19 Pandemic: AASLD Expert Panel Consensus Statement. Hepatology (Baltimore, Md.). 2020 Jul:72(1):287-304. doi: 10.1002/hep.31281. Epub
[PubMed PMID: 32298473]
Level 3 (low-level) evidence
[35]
Sultan S, Altayar O, Siddique SM, Davitkov P, Feuerstein JD, Lim JK, Falck-Ytter Y, El-Serag HB, AGA Institute. Electronic address: ewilson@gastro.org. AGA Institute Rapid Review of the Gastrointestinal and Liver Manifestations of COVID-19, Meta-Analysis of International Data, and Recommendations for the Consultative Management of Patients with COVID-19. Gastroenterology. 2020 Jul:159(1):320-334.e27. doi: 10.1053/j.gastro.2020.05.001. Epub 2020 May 11
[PubMed PMID: 32407808]
Level 1 (high-level) evidence
[36]
Parry AH, Wani AH, Yaseen M. Acute Mesenteric Ischemia in Severe Coronavirus-19 (COVID-19): Possible Mechanisms and Diagnostic Pathway. Academic radiology. 2020 Aug:27(8):1190. doi: 10.1016/j.acra.2020.05.016. Epub 2020 May 23
[PubMed PMID: 32475635]
[37]
Thompson CC, Shen L, Lee LS. COVID-19 in endoscopy: Time to do more? Gastrointestinal endoscopy. 2020 Aug:92(2):435-439. doi: 10.1016/j.gie.2020.03.3848. Epub 2020 Mar 29
[PubMed PMID: 32234312]
[38]
Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7:323(13):1239-1242. doi: 10.1001/jama.2020.2648. Epub
[PubMed PMID: 32091533]
Level 3 (low-level) evidence