Issues of Concern
Physics
Sound waves in diagnostic sonography are typically generated by piezoelectric crystals in ultrasound probes. These crystals vibrate and generate sound waves with specific frequencies when exposed to an electric current. Diagnostic sonography generally uses sound waves with a 1 to 20 MHz frequency band. As a comparison, audible sound to humans ranges from 20 Hz to 20 kHz. As sound waves propagate through tissue, they encounter various tissue interfaces. At these interfaces, sound can either be reflected, scattered, refracted, or absorbed. The loss of energy associated with this is referred to as attenuation. Higher frequencies experience greater attenuation in tissues and therefore cannot penetrate as deeply as lower frequencies.
As the sound wave travels through tissue, some echoes are reflected towards the transducer. As the sound waves return, they interact with the crystals in the ultrasound probe, causing vibration and deformation. This, in turn, causes an electric current which is relayed back to the machine. The machine is then able to process this information into an image. Because the average propagation velocity of sound waves in soft tissue is 1540 m/s, the machine uses the time it takes sound waves to return to the transducer to determine the depth of objects. Similarly, the amplitude of the waves returning to the transducer from a particular object informs the brightness of that object as displayed by the machine.[2]
Gray-scale or Brightness Mode (B-Mode) imaging utilizes the amplitude of reflected echoes to plot information into a 2-d image. Doppler ultrasonography analyzes the frequency of the returning echo to determine relative motion. The Doppler effect states that when a sonic source is moving towards or away from a stationary listening device, the relative frequency heard by the device will be shifted according to the velocity of the source. In cases where the source is moving away from the listening device, the frequency will be shifted lower, and in cases where the source is moving towards the listening device, the frequency is shifted higher. In the case of ultrasound, the transducer transmits sound waves at a given frequency to a moving object, such as a red blood cell. This object then reflects a portion of this back as an echo. As it returns to the transducer, the frequency will be altered based on the speed and direction of the traveling red blood cell. This is referred to as the Doppler frequency shift and can be calculated by:
- Fd= Ft-Fr= 2xFt x (V/c) x Cos (theta)
- Fd= Doppler frequency shift
- Ft= transmitted frequency
- Fr= received frequency
- V=Speed of moving target
- c-speed of sound in tissue
- theta = angle between flow and direction of the transmitted pulse
Because each of these elements is known except for V, the equation can be solved for the velocity of the moving reflector.
In the case of audible sounds, such as an ambulance siren or ice cream truck music, this frequency shift is perceived as a change in pitch as the source of sound travels in relation to one’s ears. In the case of ultrasound, depending on the function selected, the machine can use the frequency shift to calculate changes in velocity, which are then displayed as different shades of color or plotted out in a graphical form.
Doppler Modalities
Color
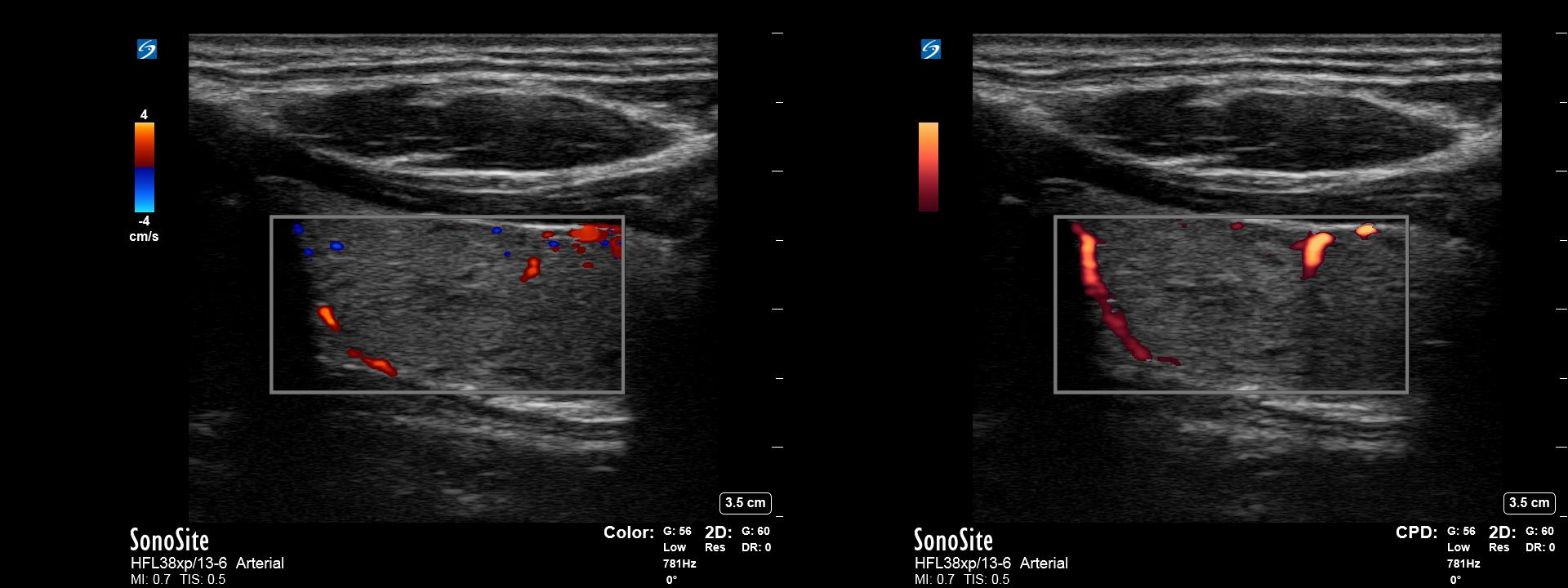
Color Doppler overlays information about the velocity of moving objects over a typical B-Mode image. In this case, a sample volume is placed over an area of interest. The machine then uses the frequency and amplitude of the returning echoes from pulsed interrogation of that region to display information regarding flow in the given area. The machine can assign different colors depending on the direction of flow, also known as phase shift. This is traditionally assigned as red for flow towards the transducer or blue for flow away from the transducer. (Figure 1) Additionally, the machine will calculate a color shade based on the mean frequency shift of an interrogated pixel, which represents the mean velocity of flow in that area. This shade is generally lighter for faster-moving objects and darker for slower-moving objects.
Color Doppler is useful to interrogate organs for the presence or absence of blood flow and quickly investigate large areas for turbulent flow. In the case of cardiac imaging, it is used to qualify regurgitant and turbulent flow. Although some quantitative information can be gained by strategic use of color Doppler, it is generally useful for qualitative information about a given sample volume.
Power
Power Doppler overlays information about the energy of returning Doppler signal instead of frequency onto a B-mode image. To do this, the transducer first obtains Doppler information identically to that in color Doppler. Using a pulsed interrogation over a sample volume, the system determines a range of echoes with shifted frequencies due to flow. However, once these shifted frequencies are determined, the machine ignores the frequency information instead of using only the amplitude information from these waves. The amplitudes are then mapped to a color (often orange) with higher power signals appearing as lighter shades. These shades represent the strength or ‘power’ of the returning signals and do not contain any information about direction or velocity. Increased strength of the signal, in this case, is attributed to an increased volume of moving blood as well as other factors such as shear rates and a number of scatterers in the sample volume.
There are several benefits to power Doppler. Power Doppler, unlike color or spectral Doppler, is less affected by doppler angle and can therefore detect flow that may be close to perpendicular to the ultrasound beam. Additionally, when increasing gain, low amplitude noise in surrounding tissue is more easily filtered out, which can increase the sensitivity of power Doppler for detecting blood flow in low flow states.[3](Figure 2)
Spectral Doppler
Spectral Doppler is a term used to describe pulsed wave (PW) Doppler and continuous wave (CW) Doppler imaging. In these forms of Doppler imaging, a range of frequencies returning to the transducer over a particular period undergo machine-automated Fast Fourier Transform functions. These functions allow averaging the returned frequencies, which are converted to velocity and plotted as a function of time.
Pulsed Wave
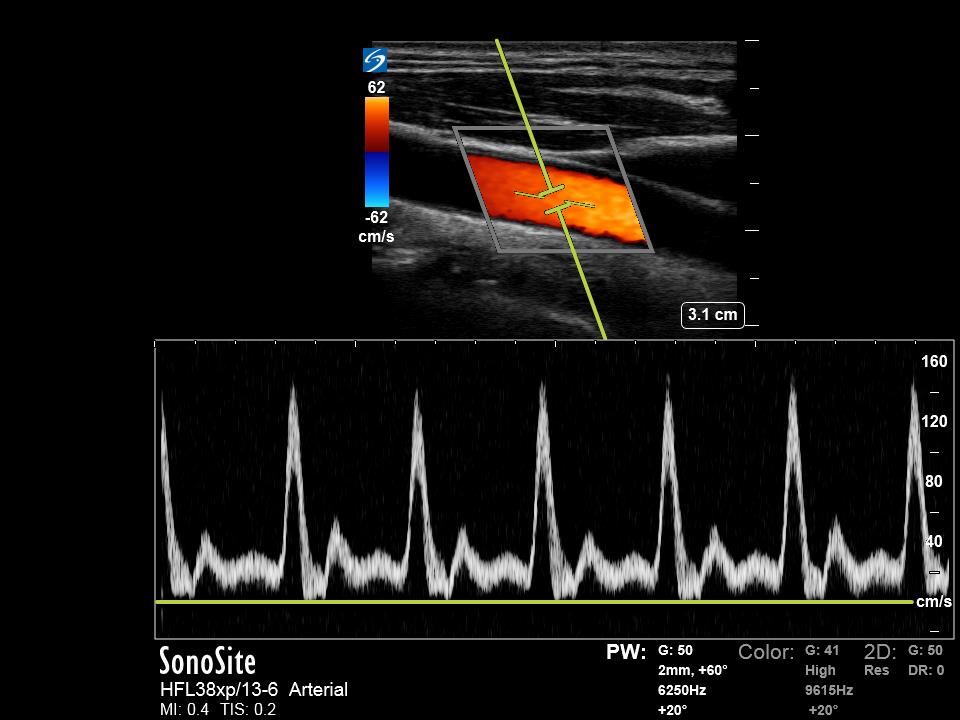
PW Doppler is used for determining frequency shifts within a specific area of interest. To do this, the user uses B-mode imaging to set the location of a sample volume or “gate” for interrogation with pulsed wave Doppler. The device then transmits short sound pulses and waits for the returning echo. Since the speed of sound in soft tissues is known, the device can vary the period during which it is ‘listening’ for returned echoes based on the expected time the sound will need to travel to return to the transducer. PW Doppler can then isolate frequency shifts from the sample volume, which is displayed in graphical form with frequency on the y-axis and time on the x-axis. If the user inputs data regarding the Doppler angle, the machine can calculate velocity and display this in place of frequency. PW Doppler waveforms typically appear “carved out” in that they only display a narrow band of velocities. This is possible because the interrogation gate can select small areas where flow velocities only exist within a narrow range. (Figure 3)
A major drawback for PW Doppler is that because of the requirement of a delay in transmission of a pulse and reception of echoes, and there is a limit to the number of pulses that can be sent over a period of time. The number of pulses sent over a period of one second is referred to as the pulse repetition frequency (PRF). The higher the velocity of the object of interest, the higher the PRF must be to accurately detect the returning frequencies. PW Doppler has a maximum limit at which the echoes from the sample volume do not have enough time to return to the transducer before the next pulse is sent. At this point, the returning echoes are no longer coordinated with the transmitted pulse, and the Doppler shift can no longer be accurately assigned. This is called an aliasing artifact and is described below.[4]
Continuous Wave
CW Doppler is used to determine frequency shifts along an entire path of interrogation. It utilizes two separate transducer elements; one transducer to transmit sound and another to receive sound. As there are separate transmission and reception transducers, sound can be transmitted and received continuously. Echoes are then filtered for those with frequency shifts, which are plotted graphically with velocity on the y-axis and time on the x-axis. Because all velocities along a given path are obtained, the waveform appears “filled-in.”
The benefit of CW Doppler is that it does not rely on pulsed sound waves. Because of this, CW Doppler can measure very high velocities. However, due to the lack of information or control regarding the depth of received frequencies, it cannot pinpoint where those velocities originated along the axis of interrogation.[5]
Image Optimization
Sample Volume Size
For pulsed Doppler modalities such as color and PW Doppler, selecting a sample volume to be interrogated is critical. Selection of color Doppler mode will present a sample box within which interrogation will be performed. The size of this box can be adjusted, although its shape will be dependent on the transducer used. Linear-style transducers will have a square or rhomboid box (if using beam steering), while phased array and curvilinear transducers will have a sector-shaped box. Interrogating a larger sample volume or larger box will result in increased processing and a lower temporal resolution (frame rate). As such, the box should be optimized to be only as large as necessary. For PW Doppler, the sample size, or “gate” size, should be adjusted to 2/3 the width of the interrogated vessel. Since the flow is typically faster in the center of a vessel than near the walls of a vessel, this minimizes the range of velocities obtained in the sample volume and will minimize spectral broadening artifacts.
Doppler Angle
In PW Doppler mode, it is important to adjust the Doppler angle of incident sound waves to optimize flow information. Because the conversion of frequency shits to velocities is dependent on the cosine of the angle of incidence, it is recommended that sonographers maintain as low a Doppler angle as possible, as this minimizes calculation error. A Doppler angle of fewer than 60 degrees is acceptable for diagnostic purposes, as small variations in angle at this level or below result in minimal changes in calculated velocity. Doppler angle correction is obtained by user input, usually by aligning a marker on the Doppler gate with the long axis of the vessel in question. Obtaining an appropriate Doppler angle is best accomplished by appropriate selection of the interrogation location, though techniques such as manual beam steering (heel/toe movements of the transducer) may assist the sonographer. In some devices, electronic beam steering can be accomplished at the transducer level. In this case, sound waves are sent out at an angle from the transducer face.[6]
Gain
Doppler gain can be adjusted separately from B-mode gain. Like B-mode gain adjustments, the adjustment of Doppler gain causes a receiver end adjustment of the amplitude of returning signals. It does not affect the properties of waves transmitted to the tissue. The goal of Doppler gain adjustment is to obtain an image with clearly visible Doppler flow in the region of interest. Adjusting the gain too high will lead to increased noise and the appearance of flow in nonvascular regions. Adjustment of gain too low will preclude visualization of flow where it is present. In practice, the best way to optimize gain is to increase it until color artifacts appear in nonvascular areas (“blooming”) and then decrease it slightly.
PRF/Scale
Adjustment of PRF or “Scale” both accomplish the same task, which is to change the velocity range, which is detectable with pulsed or color Doppler interrogation. Changing the PRF changes the number of pulses transmitted from the probe over a 1 second period. The higher the PRF, the higher the scale of velocities that can be detected. If the area of interest contains velocities higher than the upper limit of the Doppler scale, aliasing will occur, causing loss of directional and velocity information. Aliasing can occur in both color and pulsed wave Doppler modes. Increasing the PRF/Scale will improve the range of velocities able to be displayed by the device. For low velocities, lowering the scale will increase the sensitivity to low-velocity blood flow and improve the relative detection of differences in flow. In color mode, this is seen as different shades of color. In pulsed wave mode, a lower scale will reveal a larger waveform which is easier to measure accurately.
In some cases, setting the scale at a pre-determined level on color Doppler can help identify the point at which the flow characteristics change, such as at a stenotic area of a vessel. The machine will display velocities up to the limit of the set scale, at which point an area of aliasing will be apparent as velocities increase rapidly through the stenotic portion of the vessel. This can help the sonographer quickly identify the point at which velocities exceed a preset value. CW waveforms also have a scale for display purposes, and this can be adjusted to optimize the visualization of waveform details.
Wall Filter
In color and PW Doppler modes, it can be helpful to filter out low-level frequency shifts that can occur due to the movement of soft tissue/vessel walls to reduce noise on Doppler interrogation. The wall filter can be adjusted to remove these low-velocity signals in post-processing. However, as this filter is set by the user independently, it is important to note that setting the wall filter too high will remove true low-velocity flow information.
Color Assignments/Color Priority
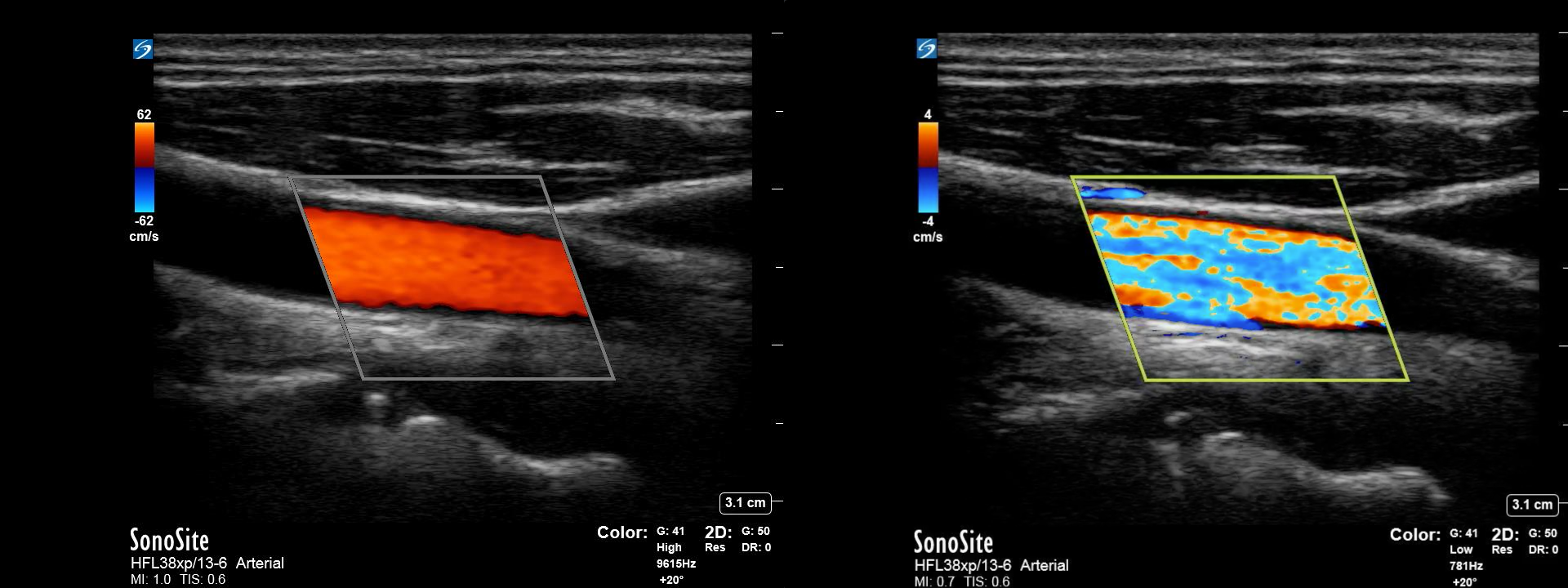
In color Doppler mode, color assignments are set and displayed as a ‘map’ on the screen. Traditionally, red is the color assigned to flow towards the transducer, and blue is assigned to flow away from the transducer. Shades of each color are then used to depict relative velocity, with lighter shades depicting faster flow in each direction. However, these color maps can be adjusted and even inverted. Additionally, color variance modes may utilize other colors to depict areas of very high velocity/turbulent flow. Color priority uses the concept that blood flow should only be present in hypoechoic or anechoic portions of the B-mode image. To prevent noise from vibrating tissue, color priority can be used to identify a level of brightness on B-mode above which color information will not be overlayed. However, this may inappropriately suppress information from vascular structures in those portions of the image.
Artifacts
Mirror
Mirror image artifacts in ultrasonography can be caused by large reflectors and large discrepancies in acoustic impedance at soft tissue interfaces. Most commonly, this can occur at interfaces near gas-containing structures such as the lung, trachea, and intestine. However, this can also occur at the fluid/soft tissue interface adjacent to vessels. Because these artifacts will also have discernable doppler flow, the sonographer needs to be aware of this possibility and adjust their technique accordingly.
Twinkle
Twinkling artifact is a specific finding which can occur when color doppler is used over a highly reflective, rough surface. Most commonly described for gallstones and renal stones, it can mimic turbulent flow or aliasing with rapid alternation of red/blue colors. Notably, this artifact will not improve when the PRF is increased. This is thought to be due to intrinsic disturbances or “noise” in doppler circuits within the machine, also known as “clock jitter.”
Aliasing
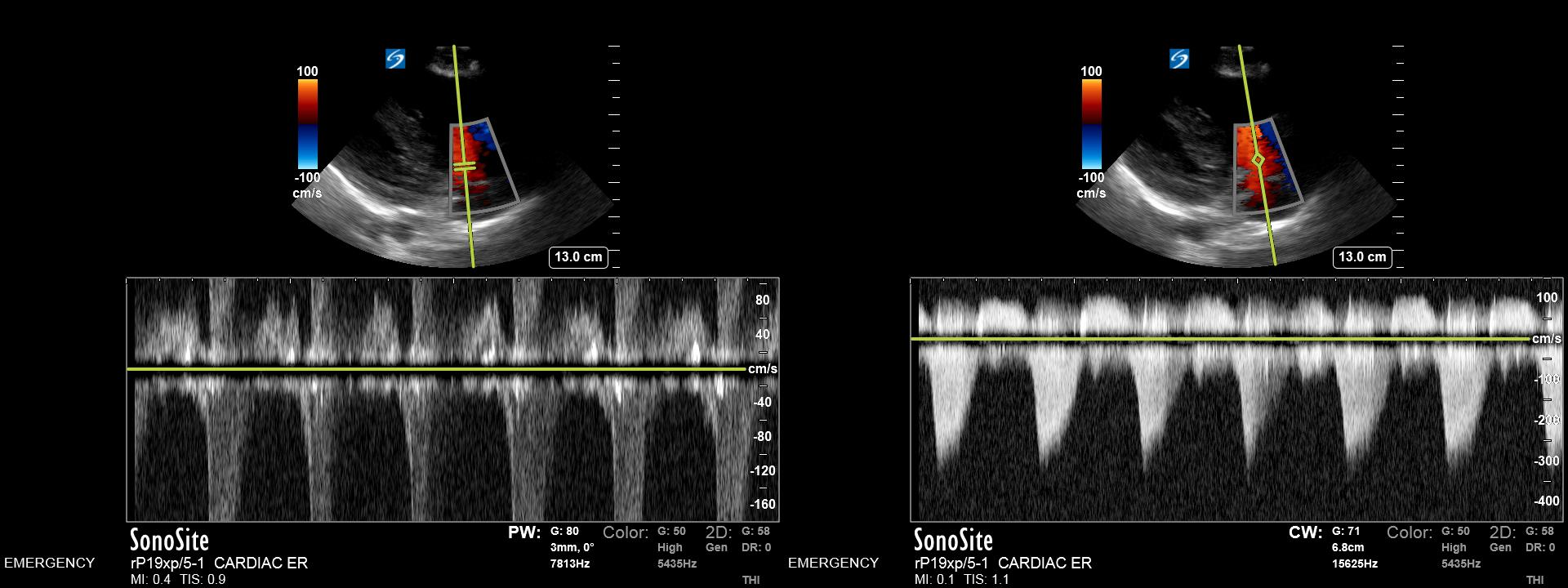
Aliasing occurs when the velocity of measured flow exceeds the limits of the set scale or PRF. The limit at which this occurs is called the Nyquist Limit and can be calculated by dividing the PRF by 2. This phenomenon can occur in both PW and color Doppler. In PW Doppler, high velocities subject to aliasing are seen to ‘wrap around’ from the highest available measured level to the bottom of the scale. In color Doppler, this appears as an abrupt change to high-velocity flow in the opposite direction. Contrasted to flow, which is truly changing direction, an area of no flow (which would be represented by an area of black) between the color boundary will not exist. Aliasing can be minimized by increasing the PRF or scale displayed by the machine, although at high velocities, it will be unavoidable. High-velocity flow that causes aliasing on the highest PRF settings, and can be aligned with low Doppler angles, such as in cardiac imaging, can sometimes be better characterized using CW Doppler mode. Additionally, because the magnitude of the Doppler frequency shift is proportional to the incident frequency, reducing the frequency of the interrogation beam may help avoid aliasing in borderline cases. (Fig 4) (Fig 5)
Blooming
Generally, due to color gain, which is set too high, blooming can occur when color information from an area containing flow spreads to contiguous areas. For example, this may appear as flow outside or along the edges of a vessel. (Fig 6)
Tissue Vibration/Transducer Movement
Movement can be transmitted to soft tissues by movement from nearby pulsating structures, such as cardiac or vascular structures. This can be picked up by Doppler imaging resulting in low-level frequency shifts in unwanted areas. Options to minimize this include adjustment of the PRF as well as wall filter and color priority settings. Transducer movement can introduce unwanted motion information, and it is imperative that sonographers attempt to minimize this movement when performing Doppler imaging. In some cases, it may be necessary to instruct patients to perform a breath-hold during the investigation to minimize this effect.[7]
Spectral Broadening
Spectral Broadening refers to the “filling in” of PW Doppler spectral waveforms due to a wide range of velocities present in the sample volume. This can occur because of a pathologic process, such as vessel stenosis, which causes artifactually disorganized and turbulent flow due to inappropriate techniques. Under normal circumstances, vascular flow is laminar, with the faster flow in the center of a vessel and relatively slower flow adjacent to the wall. In order to isolate flow in the center of the vessel for sampling, a sample Doppler gate should be sized at 2/3 the diameter of the vessel lumen and placed with a Doppler insonation angle of fewer than 60 degrees. Failure to do so may increase the range of velocities calculated by the machine and result in a wide range of velocities plotted in the waveform. (Fig 7)
Mechanical and Thermal Bioeffects
Although the use of diagnostic ultrasound is generally regarded as safe, studies have raised the possibility of harm with the unregulated use of ultrasound. There is a theoretical risk of cavitation in gaseous bodies because of the interaction between ultrasound waves and gas bodies or microbubbles within tissues. Additionally, there is a theoretical risk of thermal injury and heating of tissue due to the absorption of ultrasound energy. The energy associated with these types of injury is represented by the mechanical index (MI) and thermal index (TI) in a particular exam setting.
While these effects have not reliably been demonstrated in humans, sonography is governed by the as low as reasonably achievable (ALARA) principle regarding energy used to perform sonography. Ocular, pulmonary, and fetal tissues have the highest theoretical risk of these bioeffects, and special consideration should be taken when imaging these areas. Doppler sonography techniques have increased energy output and should only be used when necessary in susceptible tissues. As a result, the US Food and Drug Administration (FDA) and other regulatory agencies have created guidelines for using ultrasound on human tissues. The FDA and other regulatory bodies publish tables regarding suggested safe levels of MI, TI, and exposure time for different ultrasound examinations.