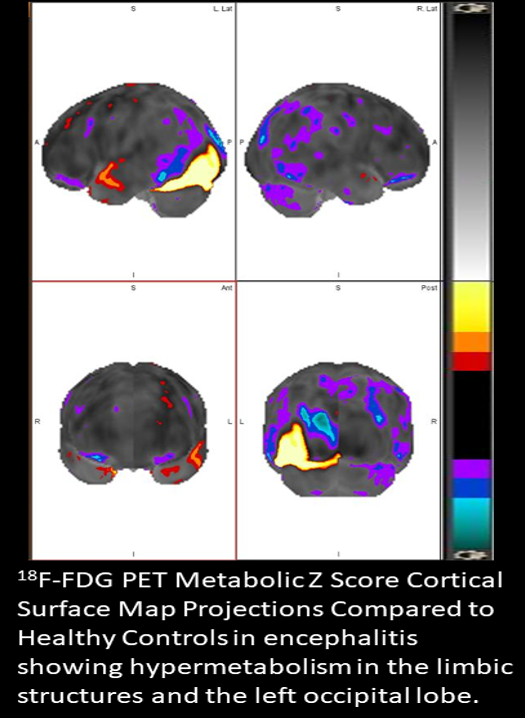
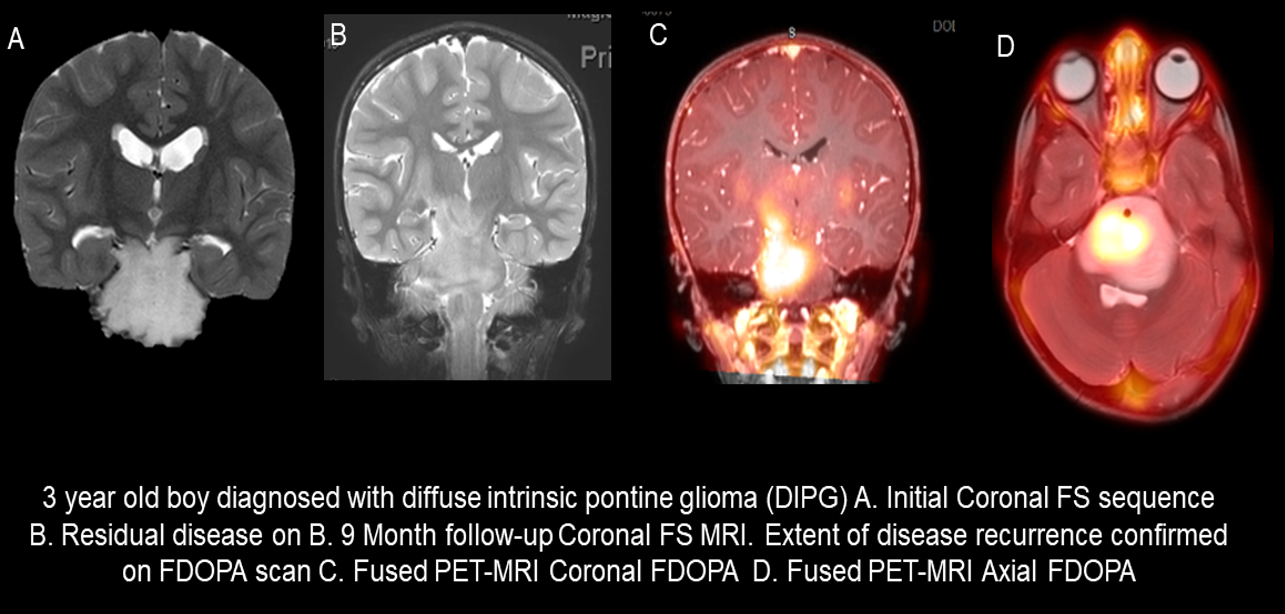
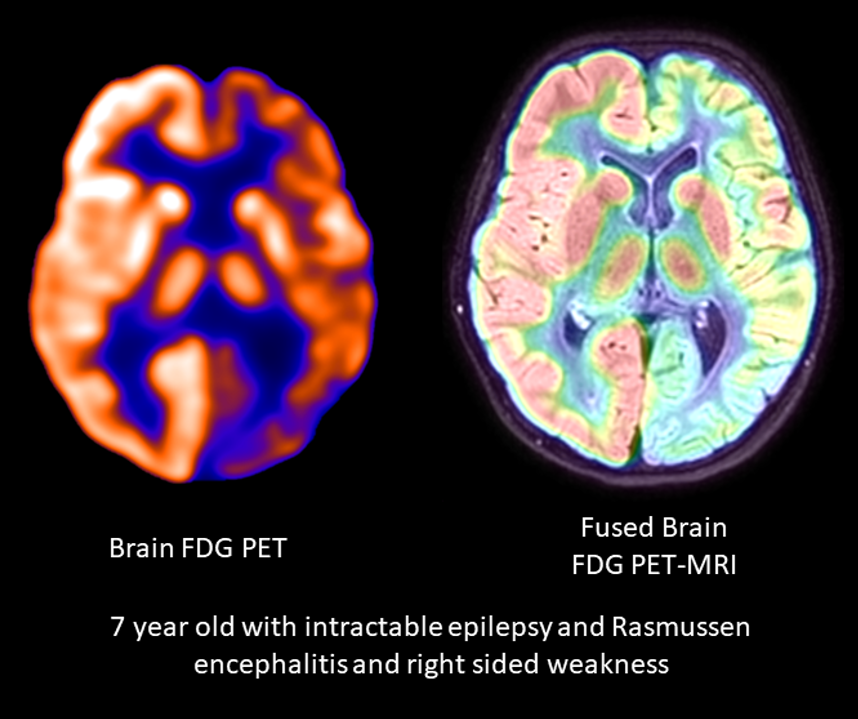
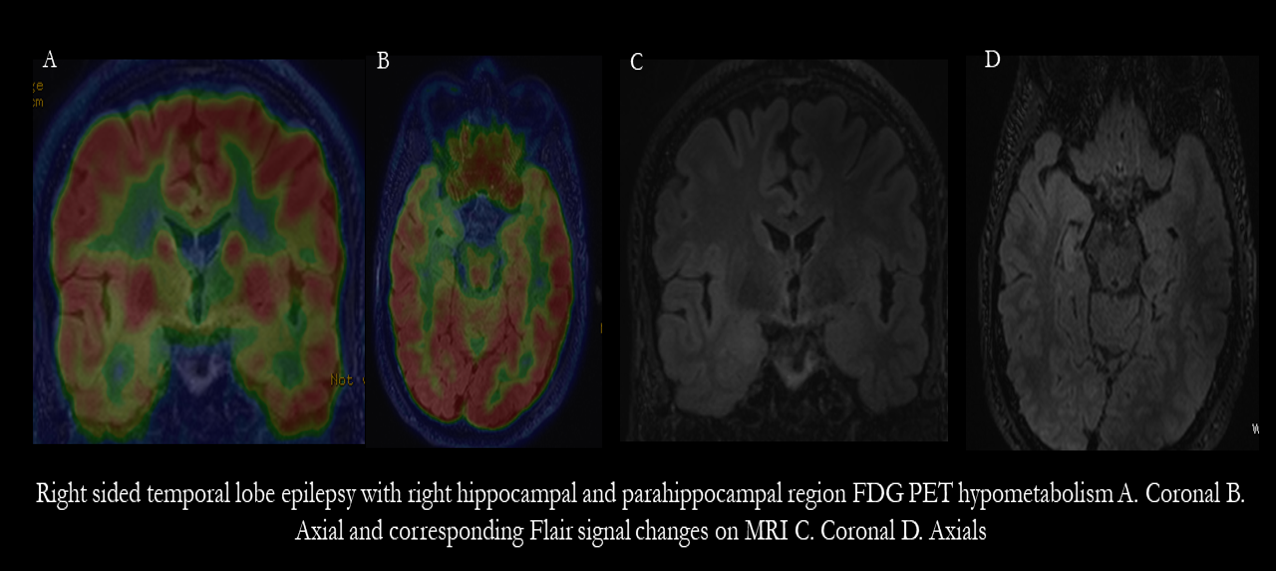
[1]
Cherry SR, Jones T, Karp JS, Qi J, Moses WW, Badawi RD. Total-Body PET: Maximizing Sensitivity to Create New Opportunities for Clinical Research and Patient Care. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2018 Jan:59(1):3-12. doi: 10.2967/jnumed.116.184028. Epub 2017 Sep 21
[PubMed PMID: 28935835]
Level 2 (mid-level) evidence
[2]
Vandenberghe S, Moskal P, Karp JS. State of the art in total body PET. EJNMMI physics. 2020 May 25:7(1):35. doi: 10.1186/s40658-020-00290-2. Epub 2020 May 25
[PubMed PMID: 32451783]
[3]
Badawi RD, Shi H, Hu P, Chen S, Xu T, Price PM, Ding Y, Spencer BA, Nardo L, Liu W, Bao J, Jones T, Li H, Cherry SR. First Human Imaging Studies with the EXPLORER Total-Body PET Scanner. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2019 Mar:60(3):299-303. doi: 10.2967/jnumed.119.226498. Epub 2019 Feb 7
[PubMed PMID: 30733314]
[4]
Bellini C, Bonioli E, Boccardo F. Lymphoscintigraphy in paediatric patients. Phlebology. 2009 Oct:24(5):237; author reply 238. doi: 10.1258/phleb.2009.009012. Epub
[PubMed PMID: 19767489]
[5]
Szuba A, Shin WS, Strauss HW, Rockson S. The third circulation: radionuclide lymphoscintigraphy in the evaluation of lymphedema. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2003 Jan:44(1):43-57
[PubMed PMID: 12515876]
[6]
Bellini C, Boccardo F, Campisi C, Villa G, Taddei G, Traggiai C, Bonioli E. Lymphatic dysplasias in newborns and children: the role of lymphoscintigraphy. The Journal of pediatrics. 2008 Apr:152(4):587-9, 589.e1-3. doi: 10.1016/j.jpeds.2007.12.018. Epub
[PubMed PMID: 18346521]
[7]
Expert Panel on Pediatric Imaging, Brown BP, Simoneaux SF, Dillman JR, Rigsby CK, Iyer RS, Alazraki AL, Bardo DME, Chan SS, Chandra T, Dorfman SR, Garber MD, Moore MM, Nguyen JC, Peters CA, Shet NS, Siegel A, Waseem M, Karmazyn B. ACR Appropriateness Criteria® Antenatal Hydronephrosis-Infant. Journal of the American College of Radiology : JACR. 2020 Nov:17(11S):S367-S379. doi: 10.1016/j.jacr.2020.09.017. Epub
[PubMed PMID: 33153550]
[8]
Lee SB, Cho YJ, Lee S, Choi YH, Cheon JE, Kim WS. Korean Society of Thyroid Radiology Guidelines for the Management of Pediatric Thyroid Nodules: Suitability and Risk Factors. Thyroid : official journal of the American Thyroid Association. 2021 Oct:31(10):1472-1480. doi: 10.1089/thy.2020.0875. Epub 2021 May 19
[PubMed PMID: 33832344]
[9]
Vali R, Alessio A, Balza R, Borgwardt L, Bar-Sever Z, Czachowski M, Jehanno N, Kurch L, Pandit-Taskar N, Parisi M, Piccardo A, Seghers V, Shulkin BL, Zucchetta P, Lim R. SNMMI Procedure Standard/EANM Practice Guideline on Pediatric (18)F-FDG PET/CT for Oncology 1.0. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2021 Jan:62(1):99-110. doi: 10.2967/jnumed.120.254110. Epub
[PubMed PMID: 33334912]
Level 1 (high-level) evidence
[10]
Majd M, Bar-Sever Z, Santos AI, De Palma D. The SNMMI and EANM Procedural Guidelines for Diuresis Renography in Infants and Children. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2018 Oct:59(10):1636-1640. doi: 10.2967/jnumed.118.215921. Epub
[PubMed PMID: 30275286]
[11]
Magill D, Alavi A. Radiation Safety Concerns Related to PET/Computed Tomography Imaging for Assessing Pediatric Diseases and Disorders. PET clinics. 2020 Jul:15(3):293-298. doi: 10.1016/j.cpet.2020.03.012. Epub
[PubMed PMID: 32498985]
[12]
Lassmann M, Treves ST, EANM/SNMMI Paediatric Dosage Harmonization Working Group. Paediatric radiopharmaceutical administration: harmonization of the 2007 EANM paediatric dosage card (version 1.5.2008) and the 2010 North American consensus guidelines. European journal of nuclear medicine and molecular imaging. 2014 May:41(5):1036-41. doi: 10.1007/s00259-014-2731-9. Epub 2014 Mar 6
[PubMed PMID: 24599377]
Level 3 (low-level) evidence
[13]
Fahey FH, Ziniel SI, Manion D, Baker A, Treves ST. Administered Activities in Pediatric Nuclear Medicine and the Impact of the 2010 North American Consensus Guidelines on General Hospitals in the United States. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016 Sep:57(9):1478-85. doi: 10.2967/jnumed.116.172148. Epub 2016 Apr 7
[PubMed PMID: 27056617]
Level 3 (low-level) evidence
[14]
Treves ST, Lassmann M, EANM/SNMMI Pediatric Dosage Harmonization Working Group. International guidelines for pediatric radiopharmaceutical administered activities. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014 Jun:55(6):869-70. doi: 10.2967/jnumed.114.139980. Epub 2014 Apr 17
[PubMed PMID: 24744446]
[15]
Ferrari C, Niccoli Asabella A, Merenda N, Altini C, Fanelli M, Muggeo P, De Leonardis F, Perillo T, Santoro N, Rubini G. Pediatric Hodgkin Lymphoma: Predictive value of interim 18F-FDG PET/CT in therapy response assessment. Medicine. 2017 Feb:96(5):e5973. doi: 10.1097/MD.0000000000005973. Epub
[PubMed PMID: 28151888]
[16]
Bakhshi S, Radhakrishnan V, Sharma P, Kumar R, Thulkar S, Vishnubhatla S, Dhawan D, Malhotra A. Pediatric nonlymphoblastic non-Hodgkin lymphoma: baseline, interim, and posttreatment PET/CT versus contrast-enhanced CT for evaluation--a prospective study. Radiology. 2012 Mar:262(3):956-68. doi: 10.1148/radiol.11110936. Epub
[PubMed PMID: 22357895]
[17]
Kluge R, Wittig T, Georgi TW, Kurch L, Sabri O, Wallace WH, Klekawka T, Fernández-Teijeiro A, Ceppi F, Karlén J, Pears J, Cepelová M, Fosså A, Beishuizen A, Hjalgrim LL, Körholz D, Mauz-Körholz C, Hasenclever D. Comparison of Interim PET Response to Second-Line Versus First-Line Treatment in Classic Hodgkin Lymphoma: Contribution to the Development of Response Criteria for Relapsed or Progressive Disease. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2021 Mar:62(3):338-341. doi: 10.2967/jnumed.120.247924. Epub 2020 Aug 6
[PubMed PMID: 32764122]
[18]
Verhagen MV, Menezes LJ, Neriman D, Watson TA, Punwani S, Taylor SA, Shankar A, Daw S, Humphries PD. (18)F-FDG PET/MRI for Staging and Interim Response Assessment in Pediatric and Adolescent Hodgkin Lymphoma: A Prospective Study with (18)F-FDG PET/CT as the Reference Standard. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2021 Nov:62(11):1524-1530. doi: 10.2967/jnumed.120.260059. Epub 2021 Feb 19
[PubMed PMID: 33608429]
[19]
Ingley KM, Nadel HR, Potts JE, Wilson DC, Eftekhari A, Deyell RJ. The Utility of PET/CT in Guiding Radiotherapy Reduction for Children With Hodgkin Lymphoma Treated With ABVD. Journal of pediatric hematology/oncology. 2020 Mar:42(2):e87-e93. doi: 10.1097/MPH.0000000000001534. Epub
[PubMed PMID: 31259825]
[20]
Rahman HA, El Semary SF, Ahmed G, Kenaai NE, Omar W, Zaky I, Nagy N. Can FDG-PET replace biopsy for the evaluation of residual tumor in pediatric mature B-cell non-Hodgkin lymphoma? Pediatric blood & cancer. 2020 Sep:67(9):e28310. doi: 10.1002/pbc.28310. Epub 2020 Jul 7
[PubMed PMID: 32634295]
[21]
Chen S, He K, Feng F, Wang S, Yin Y, Fu H, Wang H. Metabolic tumor burden on baseline (18)F-FDG PET/CT improves risk stratification in pediatric patients with mature B-cell lymphoma. European journal of nuclear medicine and molecular imaging. 2019 Aug:46(9):1830-1839. doi: 10.1007/s00259-019-04363-y. Epub 2019 Jun 11
[PubMed PMID: 31187163]
[22]
Chen S, Wang S, He K, Ma C, Fu H, Wang H. PET/CT predicts bone marrow involvement in paediatric non-Hodgkin lymphoma and may preclude the need for bone marrow biopsy in selected patients. European radiology. 2018 Jul:28(7):2942-2950. doi: 10.1007/s00330-018-5306-5. Epub 2018 Jan 30
[PubMed PMID: 29383519]
[23]
Bhojwani D, McCarville MB, Choi JK, Sawyer J, Metzger ML, Inaba H, Davidoff AM, Gold R, Shulkin BL, Sandlund JT. The role of FDG-PET/CT in the evaluation of residual disease in paediatric non-Hodgkin lymphoma. British journal of haematology. 2015 Mar:168(6):845-53. doi: 10.1111/bjh.13219. Epub 2014 Nov 10
[PubMed PMID: 25382494]
[24]
Polverari G, Ceci F, Passera R, Crane J, Du L, Li G, Fanti S, Bernthal N, Eilber FC, Allen-Auerbach M, Czernin J, Calais J, Federman N. [(18)F]FDG PET/CT for evaluating early response to neoadjuvant chemotherapy in pediatric patients with sarcoma: a prospective single-center trial. EJNMMI research. 2020 Oct 15:10(1):122. doi: 10.1186/s13550-020-00715-0. Epub 2020 Oct 15
[PubMed PMID: 33063147]
[25]
Donner D, Feraco P, Meneghello L, Rombi B, Picori L, Chierichetti F. Usefulness of 18f-FDG PET-CT in Staging, Restaging, and Response Assessment in Pediatric Rhabdomyosarcoma. Diagnostics (Basel, Switzerland). 2020 Dec 21:10(12):. doi: 10.3390/diagnostics10121112. Epub 2020 Dec 21
[PubMed PMID: 33371506]
[26]
Harrison DJ, Chi YY, Tian J, Hingorani P, Mascarenhas L, McCowage GB, Weigel BJ, Venkatramani R, Wolden SL, Yock TI, Rodeberg DA, Hayes-Jordan AA, Teot LA, Spunt SL, Meyer WH, Hawkins DS, Shulkin BL, Parisi MT. Metabolic response as assessed by (18) F-fluorodeoxyglucose positron emission tomography-computed tomography does not predict outcome in patients with intermediate- or high-risk rhabdomyosarcoma: A report from the Children's Oncology Group Soft Tissue Sarcoma Committee. Cancer medicine. 2021 Feb:10(3):857-866. doi: 10.1002/cam4.3667. Epub 2020 Dec 19
[PubMed PMID: 33340280]
[27]
El-Hennawy G, Moustafa H, Omar W, Elkinaai N, Kamel A, Zaki I, Farid N, El-Kholy E. Different (18) F-FDG PET parameters for the prediction of histological response to neoadjuvant chemotherapy in pediatric Ewing sarcoma family of tumors. Pediatric blood & cancer. 2020 Nov:67(11):e28605. doi: 10.1002/pbc.28605. Epub 2020 Jul 24
[PubMed PMID: 32706520]
[28]
Elmanzalawy A, Vali R, Chavhan GB, Gupta AA, Omarkhail Y, Amirabadi A, Shammas A. The impact of (18)F-FDG PET on initial staging and therapy planning of pediatric soft-tissue sarcoma patients. Pediatric radiology. 2020 Feb:50(2):252-260. doi: 10.1007/s00247-019-04530-1. Epub 2019 Oct 18
[PubMed PMID: 31628508]
[29]
Schmidkonz C, Krumbholz M, Atzinger A, Cordes M, Goetz TI, Prante O, Ritt P, Schaefer C, Agaimy A, Hartmann W, Rössig C, Fröhlich B, Bäuerle T, Dirksen U, Kuwert T, Metzler M. Assessment of treatment responses in children and adolescents with Ewing sarcoma with metabolic tumor parameters derived from (18)F-FDG-PET/CT and circulating tumor DNA. European journal of nuclear medicine and molecular imaging. 2020 Jun:47(6):1564-1575. doi: 10.1007/s00259-019-04649-1. Epub 2019 Dec 18
[PubMed PMID: 31853559]
[30]
El-Kholy E, El Nadi E, Hafez H, Ahmed S, Younes A, El-Kenanii N, Khalid E. Added predictive value of 18F-FDG PET/CT for pediatric rhabdomyosarcoma. Nuclear medicine communications. 2019 Sep:40(9):898-904. doi: 10.1097/MNM.0000000000001040. Epub
[PubMed PMID: 31145205]
[31]
Albano D, Dondi F, Schumacher RF, D'Ippolito C, Porta F, Giubbini R, Bertagna F. Clinical and Prognostic Role of 18F-FDG PET/CT in Pediatric Ewing Sarcoma. Journal of pediatric hematology/oncology. 2020 Mar:42(2):e79-e86. doi: 10.1097/MPH.0000000000001518. Epub
[PubMed PMID: 31135716]
[32]
Jiang M, Stanke J, Lahti JM. The connections between neural crest development and neuroblastoma. Current topics in developmental biology. 2011:94():77-127. doi: 10.1016/B978-0-12-380916-2.00004-8. Epub
[PubMed PMID: 21295685]
[33]
Vik TA, Pfluger T, Kadota R, Castel V, Tulchinsky M, Farto JC, Heiba S, Serafini A, Tumeh S, Khutoryansky N, Jacobson AF. (123)I-mIBG scintigraphy in patients with known or suspected neuroblastoma: Results from a prospective multicenter trial. Pediatric blood & cancer. 2009 Jul:52(7):784-90. doi: 10.1002/pbc.21932. Epub
[PubMed PMID: 19185008]
Level 1 (high-level) evidence
[34]
Kitamura Y, Baba S, Isoda T, Maruoka Y, Sasaki M, Kamitani T, Koga Y, Kawakubo N, Matsuura T, Ishigami K. (123)I metaiodobenzylguanidine (MIBG) uptake predicts early relapse of neuroblastoma using semi-quantitative SPECT/CT analysis. Annals of nuclear medicine. 2021 May:35(5):549-556. doi: 10.1007/s12149-021-01595-7. Epub 2021 Feb 14
[PubMed PMID: 33586098]
[35]
Piccardo A, Morana G, Puntoni M, Campora S, Sorrentino S, Zucchetta P, Ugolini M, Conte M, Cistaro A, Ferrarazzo G, Pescetto M, Lattuada M, Bottoni G, Garaventa A, Giovanella L, Lopci E. Diagnosis, Treatment Response, and Prognosis: The Role of (18)F-DOPA PET/CT in Children Affected by Neuroblastoma in Comparison with (123)I-mIBG Scan: The First Prospective Study. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2020 Mar:61(3):367-374. doi: 10.2967/jnumed.119.232553. Epub 2019 Sep 20
[PubMed PMID: 31541036]
[36]
Aboian MS, Huang SY, Hernandez-Pampaloni M, Hawkins RA, VanBrocklin HF, Huh Y, Vo KT, Gustafson WC, Matthay KK, Seo Y. (124)I-MIBG PET/CT to Monitor Metastatic Disease in Children with Relapsed Neuroblastoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2021 Jan:62(1):43-47. doi: 10.2967/jnumed.120.243139. Epub 2020 May 15
[PubMed PMID: 32414950]
[37]
Cistaro A, Quartuccio N, Caobelli F, Piccardo A, Paratore R, Coppolino P, Sperandeo A, Arnone G, Ficola U. 124I-MIBG: a new promising positron-emitting radiopharmaceutical for the evaluation of neuroblastoma. Nuclear medicine review. Central & Eastern Europe. 2015:18(2):102-6. doi: 10.5603/NMR.2015.0024. Epub
[PubMed PMID: 26315872]
[38]
Maaz AUR, O'Doherty J, Djekidel M. (68)Ga-DOTATATE PET/CT for Neuroblastoma Staging: Utility for Clinical Use. Journal of nuclear medicine technology. 2021 Sep:49(3):265-268. doi: 10.2967/jnmt.120.258939. Epub 2021 Apr 5
[PubMed PMID: 33820858]
[39]
Telli T, Lay Ergün E, Volkan Salanci B, Özgen Kiratli P. The Complementary Role of 68Ga-DOTATATE PET/CT in Neuroblastoma. Clinical nuclear medicine. 2020 Apr:45(4):326-329. doi: 10.1097/RLU.0000000000002961. Epub
[PubMed PMID: 31977455]
[40]
Marsh IR, Grudzinski J, Baiu DC, Besemer A, Hernandez R, Jeffery JJ, Weichert JP, Otto M, Bednarz BP. Preclinical Pharmacokinetics and Dosimetry Studies of (124)I/(131)I-CLR1404 for Treatment of Pediatric Solid Tumors in Murine Xenograft Models. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2019 Oct:60(10):1414-1420. doi: 10.2967/jnumed.118.225409. Epub 2019 Mar 29
[PubMed PMID: 30926646]
[41]
Lee CL, Wahnishe H, Sayre GA, Cho HM, Kim HJ, Hernandez-Pampaloni M, Hawkins RA, Dannoon SF, VanBrocklin HF, Itsara M, Weiss WA, Yang X, Haas-Kogan DA, Matthay KK, Seo Y. Radiation dose estimation using preclinical imaging with 124I-metaiodobenzylguanidine (MIBG) PET. Medical physics. 2010 Sep:37(9):4861-7
[PubMed PMID: 20964203]
[42]
Man S, Yan J, Li J, Cao Y, Hu J, Ma W, Liu J, Zhao Q. Value of pretreatment 18F-FDG PET/CT in prognosis and the reflection of tumor burden: a study in pediatric patients with newly diagnosed neuroblastoma. International journal of medical sciences. 2021:18(8):1857-1865. doi: 10.7150/ijms.58263. Epub 2021 Feb 24
[PubMed PMID: 33746603]
[43]
Sung AJ, Weiss BD, Sharp SE, Zhang B, Trout AT. Prognostic significance of pretreatment (18)F-FDG positron emission tomography/computed tomography in pediatric neuroblastoma. Pediatric radiology. 2021 Jul:51(8):1400-1405. doi: 10.1007/s00247-021-05005-y. Epub 2021 Feb 25
[PubMed PMID: 33629142]
[44]
Dadgar H, Norouzbeigi N, Ahmadzadehfar H, Assadi M. 68Ga-DOTATATE and 18F-FDG PET/CT for the Management of Esthesioneuroblastoma of the Sphenoclival Region. Clinical nuclear medicine. 2020 Aug:45(8):e363-e364. doi: 10.1097/RLU.0000000000003133. Epub
[PubMed PMID: 32558717]
[45]
Gains JE, Aldridge MD, Mattoli MV, Bomanji JB, Biassoni L, Shankar A, Gaze MN. 68Ga-DOTATATE and 123I-mIBG as imaging biomarkers of disease localisation in metastatic neuroblastoma: implications for molecular radiotherapy. Nuclear medicine communications. 2020 Nov:41(11):1169-1177. doi: 10.1097/MNM.0000000000001265. Epub
[PubMed PMID: 32796449]
[46]
Pauwels E, Celen S, Vandamme M, Leysen W, Baete K, Bechter O, Bex M, Serdons K, Van Laere K, Bormans G, Deroose CM. Improved resolution and sensitivity of [(18)F]MFBG PET compared with [(123)I]MIBG SPECT in a patient with a norepinephrine transporter-expressing tumour. European journal of nuclear medicine and molecular imaging. 2021 Jan:48(1):313-315. doi: 10.1007/s00259-020-04830-x. Epub 2020 May 8
[PubMed PMID: 32385645]
[47]
Chen H, Sippel RS, O'Dorisio MS, Vinik AI, Lloyd RV, Pacak K, North American Neuroendocrine Tumor Society (NANETS). The North American Neuroendocrine Tumor Society consensus guideline for the diagnosis and management of neuroendocrine tumors: pheochromocytoma, paraganglioma, and medullary thyroid cancer. Pancreas. 2010 Aug:39(6):775-83. doi: 10.1097/MPA.0b013e3181ebb4f0. Epub
[PubMed PMID: 20664475]
Level 3 (low-level) evidence
[48]
Ryder SJ, Love AJ, Duncan EL, Pattison DA. PET detectives: Molecular imaging for phaeochromocytomas and paragangliomas in the genomics era. Clinical endocrinology. 2021 Jul:95(1):13-28. doi: 10.1111/cen.14375. Epub 2020 Dec 9
[PubMed PMID: 33296100]
[49]
Taïeb D, Hicks RJ, Hindié E, Guillet BA, Avram A, Ghedini P, Timmers HJ, Scott AT, Elojeimy S, Rubello D, Virgolini IJ, Fanti S, Balogova S, Pandit-Taskar N, Pacak K. European Association of Nuclear Medicine Practice Guideline/Society of Nuclear Medicine and Molecular Imaging Procedure Standard 2019 for radionuclide imaging of phaeochromocytoma and paraganglioma. European journal of nuclear medicine and molecular imaging. 2019 Sep:46(10):2112-2137. doi: 10.1007/s00259-019-04398-1. Epub 2019 Jun 29
[PubMed PMID: 31254038]
Level 1 (high-level) evidence
[50]
Jha A, Ling A, Millo C, Gupta G, Viana B, Lin FI, Herscovitch P, Adams KT, Taïeb D, Metwalli AR, Linehan WM, Brofferio A, Stratakis CA, Kebebew E, Lodish M, Civelek AC, Pacak K. Superiority of (68)Ga-DOTATATE over (18)F-FDG and anatomic imaging in the detection of succinate dehydrogenase mutation (SDHx )-related pheochromocytoma and paraganglioma in the pediatric population. European journal of nuclear medicine and molecular imaging. 2018 May:45(5):787-797. doi: 10.1007/s00259-017-3896-9. Epub 2017 Dec 4
[PubMed PMID: 29204718]
[51]
Timmers HJ, Chen CC, Carrasquillo JA, Whatley M, Ling A, Havekes B, Eisenhofer G, Martiniova L, Adams KT, Pacak K. Comparison of 18F-fluoro-L-DOPA, 18F-fluoro-deoxyglucose, and 18F-fluorodopamine PET and 123I-MIBG scintigraphy in the localization of pheochromocytoma and paraganglioma. The Journal of clinical endocrinology and metabolism. 2009 Dec:94(12):4757-67. doi: 10.1210/jc.2009-1248. Epub 2009 Oct 28
[PubMed PMID: 19864450]
[52]
Jaiswal SK, Sarathi V, Malhotra G, Hira P, Shah R, Patil VA, Dalvi A, Prakash G, Lila AR, Shah NS, Bandgar T. The utility of (68)Ga-DOTATATE PET/CT in localizing primary/metastatic pheochromocytoma and paraganglioma in children and adolescents - a single-center experience. Journal of pediatric endocrinology & metabolism : JPEM. 2021 Jan 27:34(1):109-119. doi: 10.1515/jpem-2020-0354. Epub 2020 Nov 12
[PubMed PMID: 33180042]
[53]
Jaiswal SK, Sarathi V, Memon SS, Garg R, Malhotra G, Verma P, Shah R, Sehemby MK, Patil VA, Jadhav S, Lila AR, Shah NS, Bandgar TR. 177Lu-DOTATATE therapy in metastatic/inoperable pheochromocytoma-paraganglioma. Endocrine connections. 2020 Oct:9(9):864-873. doi: 10.1530/EC-20-0292. Epub
[PubMed PMID: 32784267]
[54]
Parghane RV, Talole S, Basu S. (131)I-MIBG negative progressive symptomatic metastatic paraganglioma: response and outcome with (177)Lu-DOTATATE peptide receptor radionuclide therapy. Annals of nuclear medicine. 2021 Jan:35(1):92-101. doi: 10.1007/s12149-020-01541-z. Epub 2020 Nov 1
[PubMed PMID: 33135123]
[55]
Patel M, Tena I, Jha A, Taieb D, Pacak K. Somatostatin Receptors and Analogs in Pheochromocytoma and Paraganglioma: Old Players in a New Precision Medicine World. Frontiers in endocrinology. 2021:12():625312. doi: 10.3389/fendo.2021.625312. Epub 2021 Mar 29
[PubMed PMID: 33854479]
[56]
Turnock S, Turton DR, Martins CD, Chesler L, Wilson TC, Gouverneur V, Smith G, Kramer-Marek G. (18)F-meta-fluorobenzylguanidine ((18)F-mFBG) to monitor changes in norepinephrine transporter expression in response to therapeutic intervention in neuroblastoma models. Scientific reports. 2020 Dec 1:10(1):20918. doi: 10.1038/s41598-020-77788-3. Epub 2020 Dec 1
[PubMed PMID: 33262374]
[57]
Pandit-Taskar N, Zanzonico P, Staton KD, Carrasquillo JA, Reidy-Lagunes D, Lyashchenko S, Burnazi E, Zhang H, Lewis JS, Blasberg R, Larson SM, Weber WA, Modak S. Biodistribution and Dosimetry of (18)F-Meta-Fluorobenzylguanidine: A First-in-Human PET/CT Imaging Study of Patients with Neuroendocrine Malignancies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2018 Jan:59(1):147-153. doi: 10.2967/jnumed.117.193169. Epub 2017 Jul 13
[PubMed PMID: 28705916]
[58]
Hassanzadeh-Rad A, Yousefifard M, Katal S, Asady H, Fard-Esfahani A, Moghadas Jafari A, Hosseini M. The value of (18) F-fluorodeoxyglucose positron emission tomography for prediction of treatment response in gastrointestinal stromal tumors: a systematic review and meta-analysis. Journal of gastroenterology and hepatology. 2016 May:31(5):929-35. doi: 10.1111/jgh.13247. Epub
[PubMed PMID: 26642423]
Level 1 (high-level) evidence
[59]
Rasheed R, Al-Kandari F, Ghanem M, Marafi F, Usmani S. Significance of 18F-FDG PET/CT in Characterization of Equivocal Lesions in High-Risk Testicular Carcinoma in Restaging Setting. Asian Pacific journal of cancer prevention : APJCP. 2020 Feb 1:21(2):511-515. doi: 10.31557/APJCP.2020.21.2.511. Epub 2020 Feb 1
[PubMed PMID: 32102532]
[60]
Cook GJ, Sohaib A, Huddart RA, Dearnaley DP, Horwich A, Chua S. The role of 18F-FDG PET/CT in the management of testicular cancers. Nuclear medicine communications. 2015 Jul:36(7):702-8. doi: 10.1097/MNM.0000000000000303. Epub
[PubMed PMID: 25757201]
[61]
Sanchez D, Zudaire JJ, Fernandez JM, Lopez J, Arocena J, Sanz G, Gimenez M, Rosell D, Robles JE, Berian JM. 18F-fluoro-2-deoxyglucose-positron emission tomography in the evaluation of nonseminomatous germ cell tumours at relapse. BJU international. 2002 Jun:89(9):912-6
[PubMed PMID: 12010239]
[62]
Hain SF, O'Doherty MJ, Timothy AR, Leslie MD, Partridge SE, Huddart RA. Fluorodeoxyglucose PET in the initial staging of germ cell tumours. European journal of nuclear medicine. 2000 May:27(5):590-4
[PubMed PMID: 10853816]
[63]
Sharma P, Jain TK, Parida GK, Karunanithi S, Patel C, Sharma A, Thulkar S, Julka PK, Bal C, Kumar R. Diagnostic accuracy of integrated (18)F-FDG PET/CT for restaging patients with malignant germ cell tumours. The British journal of radiology. 2014 Aug:87(1040):20140263. doi: 10.1259/bjr.20140263. Epub 2014 Jun 4
[PubMed PMID: 24896199]
[64]
Haddad T, Fard-Esfahani A, Vali R. A review of pediatric neuroendocrine tumors, their detection, and treatment by radioisotopes. Nuclear medicine communications. 2021 Jan:42(1):21-31. doi: 10.1097/MNM.0000000000001305. Epub
[PubMed PMID: 33044400]
[65]
Foster JH, Sher A, Seghers V, Poston J, Wells D, Delpassand ES, Potter S, Mahajan P, Venkatramani R. Peptide receptor radionuclide therapy for treatment of metastatic neuroendocrine tumors in children. Pediatric blood & cancer. 2021 Jul:68(7):e29056. doi: 10.1002/pbc.29056. Epub 2021 Apr 12
[PubMed PMID: 33844446]
[66]
McElroy KM, Binkovitz LA, Trout AT, Czachowski MR, Seghers VJ, Lteif AN, States LJ. Pediatric applications of Dotatate: early diagnostic and therapeutic experience. Pediatric radiology. 2020 Jun:50(7):882-897. doi: 10.1007/s00247-020-04688-z. Epub 2020 Jun 3
[PubMed PMID: 32495176]
[67]
Goel R, Shukla J, Bansal D, Sodhi K, Bhattacharya A, Marwaha RK, Mittal BR. (68)Ga-DOTATATE positron emission tomography/computed tomography scan in the detection of bone metastases in pediatric neuroendocrine tumors. Indian journal of nuclear medicine : IJNM : the official journal of the Society of Nuclear Medicine, India. 2014 Jan:29(1):13-7. doi: 10.4103/0972-3919.125762. Epub
[PubMed PMID: 24591776]
[68]
Bhavani N, Bhadran K, Nair V, Menon UV, Pavithran PV, Menon AS, Abraham N, Pankaj A, Kumar H. Treatment outcomes in pediatric differentiated thyroid carcinoma. Journal of pediatric endocrinology & metabolism : JPEM. 2018 Oct 25:31(10):1117-1122. doi: 10.1515/jpem-2018-0233. Epub
[PubMed PMID: 30157034]
[69]
Pires BP, Alves PA Jr, Bordallo MA, Bulzico DA, Lopes FP, Farias T, Dias F, Lima RA, Santos Gisler IC, Coeli CM, Carvalhaes de Oliveira RV, Corbo R, Vaisman M, Vaisman F. Prognostic Factors for Early and Long-Term Remission in Pediatric Differentiated Thyroid Carcinoma: The Role of Sex, Age, Clinical Presentation, and the Newly Proposed American Thyroid Association Risk Stratification System. Thyroid : official journal of the American Thyroid Association. 2016 Oct:26(10):1480-1487
[PubMed PMID: 27540892]
[70]
Zanella AB, Scheffel RS, Nava CF, Golbert L, Laurini de Souza Meyer E, Punales M, Gonçalves I, Dora JM, Maia AL. Dynamic Risk Stratification in the Follow-Up of Children and Adolescents with Differentiated Thyroid Cancer. Thyroid : official journal of the American Thyroid Association. 2018 Oct:28(10):1285-1292. doi: 10.1089/thy.2018.0075. Epub
[PubMed PMID: 30129889]
[71]
Lazar L, Lebenthal Y, Segal K, Steinmetz A, Strenov Y, Cohen M, Yaniv I, Yackobovitch-Gavan M, Phillip M. Pediatric Thyroid Cancer: Postoperative Classifications and Response to Initial Therapy as Prognostic Factors. The Journal of clinical endocrinology and metabolism. 2016 May:101(5):1970-9. doi: 10.1210/jc.2015-3960. Epub 2016 Mar 1
[PubMed PMID: 26930182]
[72]
Livhits MJ, Pasternak JD, Xiong M, Li N, Gosnell JE, Yeh MW, Chiu HK. PRE-ABLATION THYROGLOBULIN AND THYROGLOBULIN TO THYROID-STIMULATING HORMONE RATIO MAY BE ASSOCIATED WITH PULMONARY METASTASES IN CHILDREN WITH DIFFERENTIATED THYROID CANCER. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2016 Nov:22(11):1259-1266
[PubMed PMID: 27482611]
[73]
Quinn BM, Gao Y, Mahmood U, Pandit-Taskar N, Behr G, Zanzonico P, Dauer LT. Patient-adapted organ absorbed dose and effective dose estimates in pediatric 18F-FDG positron emission tomography/computed tomography studies. BMC medical imaging. 2020 Jan 29:20(1):9. doi: 10.1186/s12880-020-0415-4. Epub 2020 Jan 29
[PubMed PMID: 31996149]
[74]
Zampella E, Klain M, Pace L, Cuocolo A. PET/CT in the management of differentiated thyroid cancer. Diagnostic and interventional imaging. 2021 Sep:102(9):515-523. doi: 10.1016/j.diii.2021.04.004. Epub 2021 Apr 27
[PubMed PMID: 33926848]
[75]
Okuyucu K, Ince S, Alagoz E, Emer O, San H, Balkan E, Ayan A, Meric C, Haymana C, Acıkel C, Gunalp B, Karacalioglu AO, Arslan N. Risk factors and stratification for recurrence of patients with differentiated thyroid cancer, elevated thyroglobulin and negative I-131 whole-body scan, by restaging (18)F-FDG PET/CT. Hellenic journal of nuclear medicine. 2016 Sep-Dec:19(3):208-217. doi: 10.1967/s002449910402. Epub 2016 Nov 8
[PubMed PMID: 27824959]
[76]
Castinetti F, Taïeb D. Positron Emission Tomography Imaging in Medullary Thyroid Carcinoma: Time for Reappraisal? Thyroid : official journal of the American Thyroid Association. 2021 Feb:31(2):151-155. doi: 10.1089/thy.2020.0674. Epub 2020 Dec 23
[PubMed PMID: 33191866]
[77]
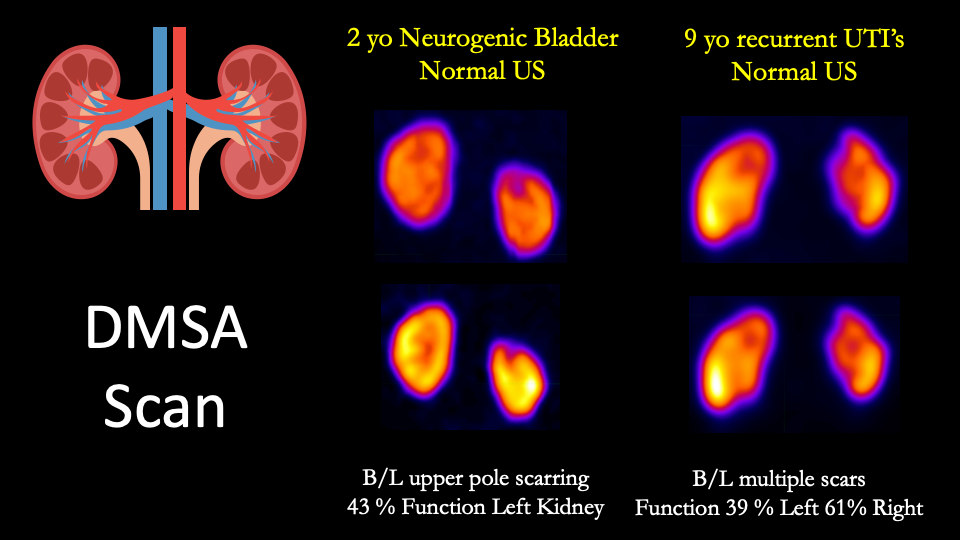
Sarikaya I, Sarikaya A. Current Status of Radionuclide Renal Cortical Imaging in Pyelonephritis. Journal of nuclear medicine technology. 2019 Dec:47(4):309-312. doi: 10.2967/jnmt.119.227942. Epub 2019 Jun 10
[PubMed PMID: 31182659]
[78]
Shaikh N, Shope TR, Hoberman A, Muniz GB, Bhatnagar S, Nowalk A, Hickey RW, Michaels MG, Kearney D, Rockette HE, Charron M, Lim R, Majd M, Shalaby-Rana E, Kurs-Lasky M, Cohen DM, Wald ER, Lockhart G, Pohl HG, Martin JM. Corticosteroids to prevent kidney scarring in children with a febrile urinary tract infection: a randomized trial. Pediatric nephrology (Berlin, Germany). 2020 Nov:35(11):2113-2120. doi: 10.1007/s00467-020-04622-3. Epub 2020 Jun 15
[PubMed PMID: 32556960]
Level 1 (high-level) evidence
[79]
Einarsdóttir HS, Berg RMG, Borgwardt L. Interrater Reliability of (99m)Tc-DMSA Scintigraphy Performed as Planar Scan vs. SPECT/Low Dose CT for Diagnosing Renal Scarring in Children. Diagnostics (Basel, Switzerland). 2020 Dec 17:10(12):. doi: 10.3390/diagnostics10121101. Epub 2020 Dec 17
[PubMed PMID: 33348530]
[80]
Ozen C, Ertan P, Aras F, Gumuser G, Ozkol M, Horasan Dinc G. Evaluation of abnormal radiological findings in children aged 2 to 36 months followed by recurrent urinary tract infection: a retrospective study. Renal failure. 2017 Nov:39(1):100-103. doi: 10.1080/0886022X.2016.1251460. Epub 2016 Nov 6
[PubMed PMID: 27819162]
Level 2 (mid-level) evidence
[81]
Roupakias S, Sinopidis X, Tsikopoulos G, Spyridakis I, Karatza A, Varvarigou A. Dimercaptosuccinic acid scan challenges in childhood urinary tract infection, vesicoureteral reflux and renal scarring investigation and management. Minerva urologica e nefrologica = The Italian journal of urology and nephrology. 2017 Apr:69(2):144-152. doi: 10.23736/S0393-2249.16.02509-1. Epub 2016 Jun 29
[PubMed PMID: 27355216]
[82]
Breinbjerg A, Jørgensen CS, Frøkiær J, Tullus K, Kamperis K, Rittig S. Risk factors for kidney scarring and vesicoureteral reflux in 421 children after their first acute pyelonephritis, and appraisal of international guidelines. Pediatric nephrology (Berlin, Germany). 2021 Sep:36(9):2777-2787. doi: 10.1007/s00467-021-05042-7. Epub 2021 Mar 23
[PubMed PMID: 33754234]
[83]
Arapović A, Punda A, Brdar D, Čapkun V, Bajo D, Veljačić D, Punda H, Simičić-Majce A, Saraga-Babić M, Vukojević K, Saraga M. Types of Parenchymal Changes Diagnosed on DMSA Scans of Kidneys Affected by Different Grades of Vesicoureteral Reflux. Medical science monitor : international medical journal of experimental and clinical research. 2021 Mar 1:27():e929617. doi: 10.12659/MSM.929617. Epub 2021 Mar 1
[PubMed PMID: 33647007]
[84]
Lee JN, Kang JK, Jeong SY, Lee SM, Cho MH, Ha YS, Kim HT, Kim TH, Yoo ES, Kwon TG, Chung SK. Predictive value of cortical transit time on MAG3 for surgery in antenatally detected unilateral hydronephrosis caused by ureteropelvic junction stenosis. Journal of pediatric urology. 2018 Feb:14(1):55.e1-55.e6. doi: 10.1016/j.jpurol.2017.08.009. Epub 2017 Sep 19
[PubMed PMID: 28988673]
[85]
Faure A, London K, Smith GH. Early mercaptoacetyltriglycine(MAG-3) diuretic renography results after pyeloplasty. BJU international. 2016 Nov:118(5):790-796. doi: 10.1111/bju.13512. Epub 2016 May 25
[PubMed PMID: 27105017]
[86]
Pui MH, Yueh TC. Lymphoscintigraphy in chyluria, chyloperitoneum and chylothorax. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998 Jul:39(7):1292-6
[PubMed PMID: 9669413]
[87]
Turpin S, Lambert R. Lymphoscintigraphy of Chylous Anomalies: Chylothorax, Chyloperitoneum, Chyluria, and Lymphangiomatosis-15-Year Experience in a Pediatric Setting and Review of the Literature. Journal of nuclear medicine technology. 2018 Jun:46(2):123-128. doi: 10.2967/jnmt.117.203281. Epub 2018 Feb 2
[PubMed PMID: 29438003]
[88]
Bellini C, Villa G, Sambuceti G, Traggiai C, Campisi C, Bellini T, Morcaldi G, Massocco D, Bonioli E, Boccardo F. Lymphoscintigraphy patterns in newborns and children with congenital lymphatic dysplasia. Lymphology. 2014 Mar:47(1):28-39
[PubMed PMID: 25109167]
[89]
Watt H, Singh-Grewal D, Wargon O, Adams S. Paediatric lymphoedema: A retrospective chart review of 86 cases. Journal of paediatrics and child health. 2017 Jan:53(1):38-42. doi: 10.1111/jpc.13305. Epub 2016 Oct 4
[PubMed PMID: 27701785]
Level 2 (mid-level) evidence
[90]
Kuo PH, Barber BJ, Kylat RI, Klewer SE, Behan S, Lau-Braunhut S, Bernas MJ, Moedano L, Bedrick AD, Mustacich DJ, Witte MH. Whole-body lymphangioscintigraphy and SPECT/CT in children with lymphatic complications after surgery for complex congenital heart disease. Lymphology. 2019:52(4):157-165
[PubMed PMID: 32171182]
[91]
Hou G, Jiang Y, Jing H, Xu W, Xu KF, Chen L, Li F, Cheng W. Usefulness of 99mTc-ASC lymphoscintigraphy and SPECT/CT in the evaluation of rare lymphatic disorders: Gorham-Stout disease, lymphangioma, and lymphangioleiomyomatosis. Medicine. 2020 Sep 25:99(39):e22414. doi: 10.1097/MD.0000000000022414. Epub
[PubMed PMID: 32991473]
[92]
Law ST, Ma KM, Li KK. The clinical characteristics of lupus related protein-losing enteropathy in Hong Kong Chinese population: 10 years of experience from a regional hospital. Lupus. 2012 Jul:21(8):840-7. doi: 10.1177/0961203312438113. Epub 2012 Feb 17
[PubMed PMID: 22343095]
[93]
Chau TN, Mok MY, Chan EY, Luk WH, Lai KB, Li FT, Leung VK, Wong R. Evaluation of performance of measurement of faecal α(1)-antitrypsin clearance and technetium-99m human serum albumin scintigraphy in protein-losing enteropathy. Digestion. 2011:84(3):199-206. doi: 10.1159/000327914. Epub 2011 Jul 8
[PubMed PMID: 21757911]
[94]
Halaby H, Bakheet SM, Shabib S, Powe JE, Al Mehaidib A, Nazer H. 99mTc-human serum albumin scans in children with protein-losing enteropathy. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2000 Feb:41(2):215-9
[PubMed PMID: 10688102]
[95]
Chiu NT, Lee BF, Hwang SJ, Chang JM, Liu GC, Yu HS. Protein-losing enteropathy: diagnosis with (99m)Tc-labeled human serum albumin scintigraphy. Radiology. 2001 Apr:219(1):86-90
[PubMed PMID: 11274540]
[97]
Stagg H, Cameron BH, Ahmed N, Butler A, Jimenez-Rivera C, Yanchar NL, Martin SR, Emil S, Anthopoulos G, Schreiber RA, Laberge JM, Canadian Biliary Atresia Registry. Variability of diagnostic approach, surgical technique, and medical management for children with biliary atresia in Canada - Is it time for standardization? Journal of pediatric surgery. 2017 May:52(5):802-806. doi: 10.1016/j.jpedsurg.2017.01.041. Epub 2017 Jan 30
[PubMed PMID: 28189446]
[98]
Shah I, Bhatnagar S, Rangarajan V, Patankar N. Utility of Tc99m-Mebrofenin hepato-biliary scintigraphy (HIDA scan) for the diagnosis of biliary atresia. Tropical gastroenterology : official journal of the Digestive Diseases Foundation. 2012 Jan-Mar:33(1):62-4
[PubMed PMID: 22803298]
[99]
Robie DK, Overfelt SR, Xie L. Differentiating biliary atresia from other causes of cholestatic jaundice. The American surgeon. 2014 Sep:80(9):827-31
[PubMed PMID: 25197861]
[100]
Jancelewicz T, Barmherzig R, Chung CT, Ling SC, Kamath BM, Ng VL, Amaral J, O'Connor C, Fecteau A, Langer JC. A screening algorithm for the efficient exclusion of biliary atresia in infants with cholestatic jaundice. Journal of pediatric surgery. 2015 Mar:50(3):363-70. doi: 10.1016/j.jpedsurg.2014.08.014. Epub
[PubMed PMID: 25746690]
[101]
Fares M, Critser PJ, Arruda MJ, Wilhelm CM, Rattan MS, Lang SM, Alsaied T. Pharmacologic stress cardiovascular magnetic resonance in the pediatric population: A review of the literature, proposed protocol, and two examples in patients with Kawasaki disease. Congenital heart disease. 2019 Nov:14(6):1166-1175. doi: 10.1111/chd.12840. Epub 2019 Sep 9
[PubMed PMID: 31498562]
[102]
Doan TT, Wilkinson JC, Loar RW, Pednekar AS, Masand PM, Noel CV. Regadenoson Stress Perfusion Cardiac Magnetic Resonance Imaging in Children With Kawasaki Disease and Coronary Artery Disease. The American journal of cardiology. 2019 Oct 1:124(7):1125-1132. doi: 10.1016/j.amjcard.2019.06.033. Epub 2019 Jul 16
[PubMed PMID: 31371063]
[103]
Abe T, Tsuda E, Sugiyama H, Kiso K, Yamada O. Risk factors of non-sustained ventricular tachycardia by technetium-perfusion imaging in patients with coronary artery lesions caused by Kawasaki disease. Journal of cardiology. 2019 May:73(5):358-362. doi: 10.1016/j.jjcc.2018.12.007. Epub 2018 Dec 31
[PubMed PMID: 30606680]
[104]
Vijarnsorn C, Noga M, Schantz D, Pepelassis D, Tham EB. Stress perfusion magnetic resonance imaging to detect coronary artery lesions in children. The international journal of cardiovascular imaging. 2017 May:33(5):699-709. doi: 10.1007/s10554-016-1041-7. Epub 2016 Dec 20
[PubMed PMID: 28000002]
[105]
Kamiyama H, Karasawa K. [Cardiac scintigraphy-Pharmacological stress and appropriate management of pediatric radiopharmaceutical administration in patients after Kawasaki disease]. Nihon rinsho. Japanese journal of clinical medicine. 2014 Sep:72(9):1595-600
[PubMed PMID: 25518408]
[106]
Abe M, Fukazawa R, Ogawa S, Watanabe M, Fukushima Y, Kiriyama T, Hayashi H, Itoh Y. Usefulness of Single Photon Emission Computed Tomography/Computed Tomography Fusion-Hybrid Imaging to Evaluate Coronary Artery Disorders in Patients with a History of Kawasaki Disease. Journal of Nippon Medical School = Nippon Ika Daigaku zasshi. 2016:83(2):71-80. doi: 10.1272/jnms.83.71. Epub
[PubMed PMID: 27180792]
[107]
Doan TT, Molossi S, Sachdeva S, Wilkinson JC, Loar RW, Weigand JD, Schlingmann TR, Reaves-O'Neal DL, Pednekar AS, Masand P, Noel CV. Dobutamine stress cardiac MRI is safe and feasible in pediatric patients with anomalous aortic origin of a coronary artery (AAOCA). International journal of cardiology. 2021 Jul 1:334():42-48. doi: 10.1016/j.ijcard.2021.04.031. Epub 2021 Apr 20
[PubMed PMID: 33892043]
[108]
Doan TT, Zea-Vera R, Agrawal H, Mery CM, Masand P, Reaves-O'Neal DL, Noel CV, Qureshi AM, Sexson-Tejtel SK, Fraser CD Jr, Molossi S. Myocardial Ischemia in Children With Anomalous Aortic Origin of a Coronary Artery With Intraseptal Course. Circulation. Cardiovascular interventions. 2020 Mar:13(3):e008375. doi: 10.1161/CIRCINTERVENTIONS.119.008375. Epub 2020 Feb 27
[PubMed PMID: 32102565]
[109]
Sinha CK, Pallewatte A, Easty M, De Coppi P, Pierro A, Misra D, Biassoni L. Meckel's scan in children: a review of 183 cases referred to two paediatric surgery specialist centres over 18 years. Pediatric surgery international. 2013 May:29(5):511-7. doi: 10.1007/s00383-013-3270-3. Epub 2013 Feb 16
[PubMed PMID: 23417523]
Level 3 (low-level) evidence
[110]
Vali R, Daneman A, McQuattie S, Shammas A. The value of repeat scintigraphy in patients with a high clinical suspicion for Meckel diverticulum after a negative or equivocal first Meckel scan. Pediatric radiology. 2015 Sep:45(10):1506-14. doi: 10.1007/s00247-015-3340-x. Epub 2015 Apr 7
[PubMed PMID: 25846077]
[111]
Dillman JR, Wong KK, Brown RK, Frey KA, Strouse PJ. Utility of SPECT/CT with Meckel's scintigraphy. Annals of nuclear medicine. 2009 Nov:23(9):813-5. doi: 10.1007/s12149-009-0301-1. Epub 2009 Sep 29
[PubMed PMID: 19784878]
[112]
Morbelli S, Djekidel M, Hesse S, Pagani M, Barthel H, Neuroimaging Committee of the European Association of Nuclear Medicine (EANM), Brain Imaging Council of the Society of Nuclear Medicine and Molecular Imaging (SNMMI). Role of (18)F-FDG-PET imaging in the diagnosis of autoimmune encephalitis. The Lancet. Neurology. 2016 Sep:15(10):1009-10. doi: 10.1016/S1474-4422(16)30140-5. Epub 2016 Aug 8
[PubMed PMID: 27571149]
[113]
Morbelli S, Arbizu J, Booij J, Chen MK, Chetelat G, Cross DJ, Djekidel M, Drzezga A, Ekmekcioglu O, Garibotto V, Hesse S, Ishii K, Jafari L, Lammertsma AA, Law I, Mathews D, Minoshima S, Mosci K, Pagani M, Pappata S, Silverman DH, Signore A, Van De Giessen E, Villemagne V, Barthel H, European Association of Nuclear Medicine (EANM) and of the Society of Nuclear Medicine and Molecular Imaging (SNMMI). The need of standardization and of large clinical studies in an emerging indication of [(18)F]FDG PET: the autoimmune encephalitis. European journal of nuclear medicine and molecular imaging. 2017 Mar:44(3):353-357. doi: 10.1007/s00259-016-3589-9. Epub 2016 Dec 6
[PubMed PMID: 27924371]
[114]
Djekidel M. (18)F-FDG PET Imaging Predicts the Epileptogenic Zone Prospectively in Recurrent Cryptogenic Meningoencephalitis with Subsequent Simple Partial Visual Seizures. Journal of nuclear medicine technology. 2021 Mar:49(1):92-94. doi: 10.2967/jnmt.120.252866. Epub 2020 Nov 20
[PubMed PMID: 33219155]
[115]
Grosse F, Wedel F, Thomale UW, Steffen I, Koch A, Brenner W, Plotkin M, Driever PH. Benefit of Static FET PET in Pretreated Pediatric Brain Tumor Patients with Equivocal Conventional MRI Results. Klinische Padiatrie. 2021 May:233(3):127-134. doi: 10.1055/a-1335-4844. Epub 2021 Feb 17
[PubMed PMID: 33598897]
[116]
Somme F, Bender L, Namer IJ, Noël G, Bund C. Usefulness of (18)F-FDOPA PET for the management of primary brain tumors: a systematic review of the literature. Cancer imaging : the official publication of the International Cancer Imaging Society. 2020 Oct 6:20(1):70. doi: 10.1186/s40644-020-00348-5. Epub 2020 Oct 6
[PubMed PMID: 33023662]
Level 1 (high-level) evidence
[117]
Tatekawa H, Yao J, Oughourlian TC, Hagiwara A, Wang C, Raymond C, Lai A, Cloughesy TF, Nghiemphu PL, Liau LM, Salamon N, Ellingson BM. Maximum Uptake and Hypermetabolic Volume of 18F-FDOPA PET Estimate Molecular Status and Overall Survival in Low-Grade Gliomas: A PET and MRI Study. Clinical nuclear medicine. 2020 Dec:45(12):e505-e511. doi: 10.1097/RLU.0000000000003318. Epub
[PubMed PMID: 33031233]
[118]
Tatekawa H, Hagiwara A, Yao J, Oughourlian TC, Ueda I, Uetani H, Raymond C, Lai A, Cloughesy TF, Nghiemphu PL, Liau LM, Pope WB, Salamon N, Ellingson BM. Voxelwise and Patientwise Correlation of (18)F-FDOPA PET, Relative Cerebral Blood Volume, and Apparent Diffusion Coefficient in Treatment-Naïve Diffuse Gliomas with Different Molecular Subtypes. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2021 Mar:62(3):319-325. doi: 10.2967/jnumed.120.247411. Epub 2020 Jul 9
[PubMed PMID: 32646876]
[119]
Qian J, Herman MG, Brinkmann DH, Laack NN, Kemp BJ, Hunt CH, Lowe V, Pafundi DH. Prediction of MGMT Status for Glioblastoma Patients Using Radiomics Feature Extraction From (18)F-DOPA-PET Imaging. International journal of radiation oncology, biology, physics. 2020 Dec 1:108(5):1339-1346. doi: 10.1016/j.ijrobp.2020.06.073. Epub 2020 Jul 4
[PubMed PMID: 32634544]
Level 2 (mid-level) evidence
[120]
Castello A, Riva M, Fernandes B, Bello L, Lopci E. The role of 11C-methionine PET in patients with negative diffusion-weighted magnetic resonance imaging: correlation with histology and molecular biomarkers in operated gliomas. Nuclear medicine communications. 2020 Jul:41(7):696-705. doi: 10.1097/MNM.0000000000001202. Epub
[PubMed PMID: 32371671]
[121]
Spies AJ, Steyn M, Bussy E, Brits D. Forensic imaging: The sensitivities of various imaging modalities in detecting skeletal trauma in simulated cases of child abuse using a pig model. Journal of forensic and legal medicine. 2020 Nov:76():102034. doi: 10.1016/j.jflm.2020.102034. Epub 2020 Aug 6
[PubMed PMID: 33208232]
Level 3 (low-level) evidence
[122]
Chuang YW, Hsu CC, Chang CC, Lin CY, Chu HL, Huang YF, Tyan YC. Multiple Bony Injuries on Bone Scan in a Case of Unsuspected Child Abuse. Case reports in medicine. 2017:2017():3015941. doi: 10.1155/2017/3015941. Epub 2017 Jun 27
[PubMed PMID: 28740509]
Level 3 (low-level) evidence
[123]
Born M, Schwier F, Stoever B, Mentzel HJ, Freiberg J. The German Evidence-Based Child Protection Guideline - Imaging in Suspected Child Abuse. RoFo : Fortschritte auf dem Gebiete der Rontgenstrahlen und der Nuklearmedizin. 2020 Apr:192(4):343-348. doi: 10.1055/a-1019-8018. Epub 2019 Nov 20
[PubMed PMID: 31747703]
[124]
Bainbridge JK, Huey BM, Harrison SK. Should bone scintigraphy be used as a routine adjunct to skeletal survey in the imaging of non-accidental injury? A 10 year review of reports in a single centre. Clinical radiology. 2015 Aug:70(8):e83-9. doi: 10.1016/j.crad.2015.04.012. Epub 2015 Jun 6
[PubMed PMID: 26055408]
Level 3 (low-level) evidence
[125]
Drubach LA. Nuclear Medicine Techniques in Pediatric Bone Imaging. Seminars in nuclear medicine. 2017 May:47(3):190-203. doi: 10.1053/j.semnuclmed.2016.12.006. Epub 2017 Jan 12
[PubMed PMID: 28417851]
[126]
Valanne L, Föhr A. [Radiological examinations in suspected physical abuse of a child]. Duodecim; laaketieteellinen aikakauskirja. 2015:131(10):1000-7
[PubMed PMID: 26237881]
[127]
Grant FD. ¹⁸F-fluoride PET and PET/CT in children and young adults. PET clinics. 2014 Jul:9(3):287-97. doi: 10.1016/j.cpet.2014.03.004. Epub
[PubMed PMID: 25030392]
[128]
Niccoli Asabella A, Stabile Ianora AA, Di Palo A, Rubini D, Pisani AR, Ferrari C, Notaristefano A, Rubini G. [Lung perfusion scintigraphy in pediatric patients with congenital malformations]. Recenti progressi in medicina. 2013 Jul-Aug:104(7-8):442-5. doi: 10.1701/1315.14593. Epub
[PubMed PMID: 24042425]
[129]
Wise-Faberowski L, Irvin M, Lennig M, Long J, Nadel HR, Bauser-Heaton H, Asija R, Hanley FL, McElhinney DB. Assessment of the Reconstructed Pulmonary Circulation With Lung Perfusion Scintigraphy After Unifocalization and Repair of Tetralogy of Fallot With Major Aortopulmonary Collaterals. World journal for pediatric & congenital heart surgery. 2019 May:10(3):313-320. doi: 10.1177/2150135119836735. Epub
[PubMed PMID: 31084304]
[130]
Asabella AN, Cimino A, Altini C, Lavelli V, Rubini G. Lung Perfusion Imaging in Tetralogy of Fallot: A Case Report. Molecular imaging and radionuclide therapy. 2018 Oct 9:27(3):146-148. doi: 10.4274/mirt.04909. Epub
[PubMed PMID: 30317856]
Level 3 (low-level) evidence
[131]
Chien KJ, Huang HW, Huang TC, Lee CL, Weng KP, Lin CC, Shieh PC, Wu MT, Hsieh KS. Assessment of branch pulmonary artery stenosis in children after repair of tetralogy of Fallot using lung perfusion scintigraphy comparison with echocardiography. Annals of nuclear medicine. 2016 Jan:30(1):49-59
[PubMed PMID: 26493388]
[132]
Rubió Rodríguez A, Ferran Sureda N, Balliu Collgròs E, Uriel Prat S, Galofré Mora P. [Unilateral ventilation/perfusión (V/Q) mismatch in a child with corrected congenital heart disease]. Revista espanola de medicina nuclear. 2009 Jan-Feb:28(1):30-1
[PubMed PMID: 19232176]
[133]
Kamal A, Sarvepalli S, Selvakumar P, Lopez R, Radhakrishnan K, Gabbard S. Assessment of Gastric Emptying Times Between Pediatrics and Adults With Cyclic Vomiting Syndrome. Journal of clinical gastroenterology. 2020 Oct:54(9):e89-e92. doi: 10.1097/MCG.0000000000001352. Epub
[PubMed PMID: 32569030]
[134]
Kwatra NS, Shalaby-Rana E, Andrich MP, Tsai J, Rice AL, Ghelani SJ, Spottswood SE, Majd M. Gastric emptying of milk in infants and children up to 5 years of age: normative data and influencing factors. Pediatric radiology. 2020 May:50(5):689-697. doi: 10.1007/s00247-020-04614-3. Epub 2020 Jan 28
[PubMed PMID: 31993707]
[135]
Edwards ST, Cocjin J, Theut SB, Rivard D, Sherman AK, Friesen CA. A comparison of the diagnosis of gastroparesis in 4 h pediatric gastric emptying studies versus 2 h studies. BMC gastroenterology. 2019 Feb 11:19(1):26. doi: 10.1186/s12876-019-0948-6. Epub 2019 Feb 11
[PubMed PMID: 30744574]
[136]
Du T, Hill L, Ding L, Towbin AJ, DeJonckheere M, Bennett P, Hagerman N, Varughese AM, Pratap JN. Gastric emptying for liquids of different compositions in children. British journal of anaesthesia. 2017 Nov 1:119(5):948-955. doi: 10.1093/bja/aex340. Epub
[PubMed PMID: 29077812]
[137]
Wu H, Zhao R. Image characteristics and classification of salivagram in the diagnosis of pulmonary aspiration in children. Nuclear medicine communications. 2017 Jul:38(7):617-622. doi: 10.1097/MNM.0000000000000688. Epub
[PubMed PMID: 28471844]
[138]
Shao F, Zhao X, Toyama H, Ichihara T, Zhuang H, Zhao R, Kung BT, Ng KS, Zhang Z, Wu H. Semi-quantitative assessment optimized the grading of pulmonary aspiration on salivagram in children. Annals of nuclear medicine. 2021 Mar:35(3):321-327. doi: 10.1007/s12149-020-01564-6. Epub 2021 Feb 8
[PubMed PMID: 33555558]
[139]
Kim GE, Sung IY, Ko EJ, Choi KH, Kim JS. Comparison of Videofluoroscopic Swallowing Study and Radionuclide Salivagram for Aspiration Pneumonia in Children With Swallowing Difficulty. Annals of rehabilitation medicine. 2018 Feb:42(1):52-58. doi: 10.5535/arm.2018.42.1.52. Epub 2018 Feb 28
[PubMed PMID: 29560324]
[140]
Wu H, Zhao X, Ting Kung B, Sing Ng K. Effect of nasogastric tube on salivagram result in paediatric patients. Nuclear medicine communications. 2019 Sep:40(9):894-897. doi: 10.1097/MNM.0000000000001052. Epub
[PubMed PMID: 31343616]
[141]
Li Q, Tian R, Sun X. More Evidence Is Warranted to Establish the Role of 18FDG-PET/CT in Fever of Unknown Origin (FUO) Investigations Among Children. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2021 Nov 2:73(9):e2842-e2844. doi: 10.1093/cid/ciaa1574. Epub
[PubMed PMID: 33064795]
[142]
Pijl JP, Kwee TC, Legger GE, Peters HJH, Armbrust W, Schölvinck EH, Glaudemans AWJM. Role of FDG-PET/CT in children with fever of unknown origin. European journal of nuclear medicine and molecular imaging. 2020 Jun:47(6):1596-1604. doi: 10.1007/s00259-020-04707-z. Epub 2020 Feb 7
[PubMed PMID: 32030452]
[143]
Shimizu M, Ikawa Y, Mizuta M, Takakura M, Inoue N, Nishimura R, Yachie A. FDG-PET in macrophage activation syndrome associated with systemic juvenile idiopathic arthritis. Pediatrics international : official journal of the Japan Pediatric Society. 2017 Apr:59(4):509-511. doi: 10.1111/ped.13238. Epub
[PubMed PMID: 28401744]
[144]
Shimizu M, Saikawa Y, Yachie A. Role of 18-fluoro-2-deoxyglucose positron emission tomography in detecting acute inflammatory lesions of non-bacterial osteitis in patients with a fever of unknown origin: A comparative study of 18-fluoro-2-deoxyglucose positron emission tomography, bone scan, and magnetic resonance imaging. Modern rheumatology. 2018 Nov:28(6):1058-1062. doi: 10.1080/14397595.2016.1193112. Epub 2016 Jun 20
[PubMed PMID: 27321232]
Level 2 (mid-level) evidence
[145]
Garg G, DaSilva R, Bhalakia A, Milstein DM. Utility of fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography in a child with chronic granulomatous disease. Indian journal of nuclear medicine : IJNM : the official journal of the Society of Nuclear Medicine, India. 2016 Jan-Mar:31(1):62-4. doi: 10.4103/0972-3919.172366. Epub
[PubMed PMID: 26917900]
[146]
Chang L, Cheng MF, Jou ST, Ko CL, Huang JY, Tzen KY, Yen RF. Search of Unknown Fever Focus Using PET in Critically Ill Children With Complicated Underlying Diseases. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2016 Feb:17(2):e58-65. doi: 10.1097/PCC.0000000000000601. Epub
[PubMed PMID: 26649939]
[147]
Jiménez D, Soto ME, Martínez-Martínez LA, Hernández-López D, Lerma C, Barragán-Garfias JA, Pérez-Torres I, Hernández-Lemus E, Guarner-Lans V. Assessment of inflammatory activity in Takayasu's arteritis: performance of clinical scores and common biomarkers versus 18F-FDG PET/CT. Clinical and experimental rheumatology. 2021 Sep-Oct:39(5):1011-1020. doi: 10.55563/clinexprheumatol/a71bzh. Epub 2020 Oct 29
[PubMed PMID: 33124558]
[148]
Kawamura J, Ueno K, Taimura E, Matsuba T, Imoto Y, Jinguji M, Kawano Y. Case Report: (18)F-FDG PET-CT for Diagnosing Prosthetic Device-Related Infection in an Infant With CHD. Frontiers in pediatrics. 2021:9():584741. doi: 10.3389/fped.2021.584741. Epub 2021 Mar 8
[PubMed PMID: 33763393]
Level 3 (low-level) evidence
[149]
Aslanidis IP, Pursanova DM, Mukhortova OV, Shurupova IV, Ekaeva IV, Arakelyan VS, Golukhova EZ, Mironenko VA, Garmanov SV, Popov DA. [18F-fluorodeoxyglucose PET/CT in the diagnosis of vascular graft infection]. Khirurgiia. 2021:(2):58-66. doi: 10.17116/hirurgia202102158. Epub
[PubMed PMID: 33570356]
[150]
Cremer PC. Diagnostic Uncertainty in Prosthetic Valve Endocarditis: Value of (18)F-FDG PET/CT and the Need for Standardization. JACC. Cardiovascular imaging. 2020 Dec:13(12):2616-2618. doi: 10.1016/j.jcmg.2020.06.023. Epub 2020 Aug 19
[PubMed PMID: 32828768]
[151]
Jerónimo A, Olmos C, Vilacosta I, Ortega-Candil A, Rodríguez-Rey C, Pérez-Castejón MJ, Fernández-Pérez C, Pérez-García CN, García-Arribas D, Ferrera C, Carreras JL. Accuracy of (18)F-FDG PET/CT in patients with the suspicion of cardiac implantable electronic device infections. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2022 Apr:29(2):594-608. doi: 10.1007/s12350-020-02285-z. Epub 2020 Aug 3
[PubMed PMID: 32748277]
[152]
Gobbo MY, Meretta AH, Rosa D, Corneli M, Daquarti GJ, Masoli OH, Grymberg L, Pérez Baliño N, Nacinovich F, Ronderos R. [Positron emission tomography in infective endocarditis associated with intracardiac devices and prosthetic valves]. Medicina. 2020:80(1):17-22
[PubMed PMID: 32044737]
[153]
Treglia G. Diagnostic Performance of (18)F-FDG PET/CT in Infectious and Inflammatory Diseases according to Published Meta-Analyses. Contrast media & molecular imaging. 2019:2019():3018349. doi: 10.1155/2019/3018349. Epub 2019 Jul 25
[PubMed PMID: 31427907]
[154]
Chen W, Sajadi MM, Dilsizian V. Merits of FDG PET/CT and Functional Molecular Imaging Over Anatomic Imaging With Echocardiography and CT Angiography for the Diagnosis of Cardiac Device Infections. JACC. Cardiovascular imaging. 2018 Nov:11(11):1679-1691. doi: 10.1016/j.jcmg.2018.08.026. Epub
[PubMed PMID: 30409329]
[155]
Diemberger I, Bonfiglioli R, Martignani C, Graziosi M, Biffi M, Lorenzetti S, Ziacchi M, Nanni C, Fanti S, Boriani G. Contribution of PET imaging to mortality risk stratification in candidates to lead extraction for pacemaker or defibrillator infection: a prospective single center study. European journal of nuclear medicine and molecular imaging. 2019 Jan:46(1):194-205. doi: 10.1007/s00259-018-4142-9. Epub 2018 Sep 8
[PubMed PMID: 30196365]
[156]
Blanc P, Bonnet E, Giordano G, Monteil J, Salabert AS, Payoux P. The use of labelled leucocyte scintigraphy to evaluate chronic periprosthetic joint infections: a retrospective multicentre study on 168 patients. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2019 Sep:38(9):1625-1631. doi: 10.1007/s10096-019-03587-y. Epub 2019 Jun 20
[PubMed PMID: 31218592]
Level 2 (mid-level) evidence
[157]
Djekidel M, Brown RK, Piert M. Benefits of hybrid SPECT/CT for (111)In-oxine- and Tc-99m-hexamethylpropylene amine oxime-labeled leukocyte imaging. Clinical nuclear medicine. 2011 Jul:36(7):e50-6. doi: 10.1097/RLU.0b013e31821738a0. Epub
[PubMed PMID: 21637042]