Continuing Education Activity
Dementia describes an overall decline in memory and other cognitive skills severe enough to reduce a person's ability to perform everyday activities. It is characterized by the progressive and persistent deterioration of cognitive function. Affected patients often have memory loss and a partial or significant lack of insight into their deficits. This activity reviews the evaluation and management of dementia and highlights the role of the interprofessional team in caring for patients with this condition. It also reviews risk factors, pathophysiology, and challenges in diagnosing and managing this spectrum of cognitive disorders.
Objectives:
Describe risk factors associated with dementia and related conditions.
Review the evaluation required for patients presenting with cognitive decline.
Summarize the commonly available management options available for patients with various cognitive decline syndromes.
Outline the importance of improving care coordination among the interprofessional team to enhance the delivery of care and improve outcomes for patients affected by dementia.
Introduction
The definition of dementia has been updated in the DSM-5 criteria. It is actually no longer termed Dementia but is now called Major Neurocognitive Disorder (MND). However, due to the common use of the term dementia in society and medical literature, it will be referred to as both Dementia and Major Neurocognitive Disorder in this article. It is worth noting the limitations of using the term dementia, including its common association exclusively with older patients, and that it is often used synonymously with Alzheimer disease. Major neurocognitive disorder can affect younger individuals and does not always imply Alzheimer disease as the etiology of cognitive decline. Major neurocognitive disorder is characterized by a significant decline in at least one of the domains of cognition which include executive function, complex attention, language, learning, memory, perceptual-motor, or social cognition. The decline represents a change from a patient's prior level of cognitive ability, is persistent and progressive over time, and is not associated exclusively with an episode of delirium. In addition to the cognitive decline, there must also be a decline in the patient's ability to function and perform everyday tasks. The everyday function of a patient is often evaluated in terms of the ability to perform IADLs (Instrumental Activities of Daily Living), such as managing finances or medications, or, if more severe, ADLs (Activities of Daily Living), such as grooming or feeding oneself.[1] It is often a progressive disorder, and individuals often do not have insight into their deficits. Currently, no cure exists for any of the causes of dementia. The prevalence of dementia is expected to continue to increase along with the increasing numbers of the aging population. Currently, 47 million people in the world have dementia, and the number is expected to increase to 131 million by 2050.[2] Alzheimer disease is the 5th leading cause of death for people over the age of 65 in the United States.[3] Dementia is a significant public health burden and significantly increases the costs of care, both to the individual and society. The individual lifetime cost to care for an individual with dementia was nearly $200,000 more than an individual without dementia.[4] In 2010, the costs of treating dementia in the United States were projected to be about $200 billion per year in the United States and $600 billion worldwide. [5]
Etiology
Several conditions can cause major neurocognitive disorder with Alzheimer dementia (AD) being the most common cause accounting for about 70% of cases.[6] The DSM-5 criteria for major neurocognitive disorder further delineate 13 etiological subtypes that indicate the possible etiology of the disorder. These subtypes include Alzheimer disease, vascular disease, frontotemporal lobar degeneration, Lewy body disease, Parkinson disease, HIV infection, Huntington disease, prion disease, substance and or medication use, traumatic brain injury, another medical condition, multiple etiologies, and unspecified. A patient may have more than one etiology contributing to MND. For example, there may be a mixed picture of Alzheimer disease with vascular disease in the same patient. Other medical conditions that can lead to dementia include progressive supranuclear palsy, corticobasal syndrome, and, less commonly, multiple system atrophy. The etiology is further characterized by "possibly" vs. "probably," assigning the degree of certainty as to the cause of the major neurocognitive disorder. It often takes time to distinguish the etiology and can be aided by many factors, including the results of imaging studies, lab studies, genetic markers, patient comorbidities, medical and family history, and clinical findings.[1]
Epidemiology
Alzheimer disease is the most common cause of dementia, as it is responsible for 70 to 80% of all cases of dementia. It can occur sporadically or be familial.[7]
Vascular dementia accounts for approximately 15% of all dementia cases. Its incidence increases with age and doubles every 5.3 years. Risk factors for vascular dementia include hypercholesteremia, diabetes mellitus, hypertension, and smoking.[8]
Lewy body dementia accounts for approximately 5% of dementia cases. The epidemiological data may not be completely accurate because the diagnosis of Lewy boy dementia is often missed.[9]
Parkinson disease dementia accounts for approximately 10% of cases of dementia. [9]
Frontotemporal dementia is attributed to 25% of dementia cases in patients older than 65 years of age. It is, however, the second most common cause of dementia in patients younger than 65 years. There are, however, many limitations in the epidemiological studies of frontotemporal dementia, in part due to the inherent difficulty in identifying frontotemporal dementia.[10]
Creutzfeldt-Jakob disease is rare and occurs in about 1 to 2 cases per million per year globally.[11]
Mixed dementia is a condition in which patients have more than one type of dementia. In this condition, Alzheimer disease with vascular dementia is the most common coexistent dementia.[12]
Pathophysiology
The pathophysiology of major neurocognitive disorder, or dementia, varies depending on the subtype. Most types of dementia, except vascular dementia, are caused by the accumulation of native proteins in the brain.
Alzheimer disease is characterized by widespread atrophy of the cortex and deposition of amyloid plaques, and neurofibrillary tangles of hyperphosphorylated tau protein in neurons which contribute to their degeneration.[7]
Lewy body dementia and Parkinson disease dementia are characterized by the intracellular accumulation of Lewy bodies, which are insoluble aggregates of alpha-synuclein protein in the brain.[13]
Frontotemporal dementia is characterized by various mutations leading to the deposition of ubiquitinated TDP-43 and hyperphosphorylated tau proteins in the frontal and temporal lobes leading to dementia, early personality, behavioral changes, and aphasia depending on the subtype.[10]
Huntington disease is caused by an autosomal dominant inherited gene mutation. [14]
Prion-related dementias are caused by misfolded prions, which are proteinaceous particles that are infectious in nature and self-spreading. Prion dementias include Creutzfeldt-Jakob disease and kuru, among other syndromes.[15]
HIV infection is associated with the development of neurocognitive disorders, in part due to the activation of macrophages and toxic inflammation leading to neurodegeneration in the brain.[16]
Alcohol consumption, particularly high dose and prolonged use, is associated with multiple cytotoxic processes within the brain.[17]
Vascular dementia is caused by ischemic injury to the brain (e.g., stroke), leading to permanent neuronal death.[18]
Histopathology
The pathological changes in the brain of patients with different types of dementia can be varied. However, there is often overlap and mixed presentations and findings.
Neurodegeneration and vascular changes are seen in the brains of patients with vascular dementia. The findings can vary and are related to the underlying etiology of vascular compromise, including lacunar infarcts, hemorrhagic lesions, and microvascular disease.[19]
There is considerable overlap of neuropathological findings between dementia with Lewy body and Parkinson disease, and some overlap with Alzheimer disease. Lewy body dementia and Parkinson disease are characterized by the presence of Lewy bodies throughout the neocortex, brainstem, and limbic regions of the brain. Lewy bodies are intracellular aggregates of proteins, predominantly composed of alpha-synuclein proteins, which can be highlighted by various stains depending on location within the brain. There is also a loss of midbrain dopaminergic neurons and a loss of cholinergic neurons in ventral forebrain nuclei. Additionally, there are neuritic plaques made up of amyloid and neurofibrillary tangles, which can overlap findings in AD.[20]
Alzheimer disease is characterized by neuritic plaques composed of extracellular amyloid beta protein deposition and neurofibrillary tangles composed of hyperphosphorylated tau proteins. It is also common to see signs of vascular ischemic damage and hippocampal sclerosis.[21][22]
Frontotemporal dementia is characterized by atrophy in the frontal and or temporal lobes. There is neuronal loss, microvacuolation, and loss of myelin. Degeneration is found in the cortical and basal ganglia. There are four different pathological subtypes named after the proteins that make up inclusions found in the brain tissue. FTLD-tau and FTLD-TDP are the most common. FTLD-FET is less common, and FTD-UPS is quite rare.[23]
Cruetzfield Jakob disease often does not need an autopsy for diagnosis; however, it will show loss of neurons, spongiform degeneration (vacuoles in the intraneuronal space), or plaques positive for PrPSc.[24]
History and Physical
History must be obtained from the patient and their close friends, family members, or caregivers. Patients may present with symptoms of changes in behavior, getting lost in familiar neighborhoods, memory loss, mood changes, aggression, social withdrawal, self-neglect, cognitive difficulty, personality changes, difficulty performing tasks, forgetfulness, difficulty in communication, loss of independence, etc. A detailed history should include past medical, family, medication, and substance use history and defining observed symptoms of cognitive decline. Often patients will report different awareness of the deficits than caregivers or companions will report. In addition to further characterization of the cognitive changes, it is important to evaluate their current functional abilities and any changes in their ability to perform daily tasks.
It is also vitally important to evaluate any safety concerns that arise from the cognitive changes. For example, is the patient still driving, and are they doing so safely? Have there been any episodes of wandering or getting lost? If there was a fire in the house, could they get out safely? Can they still use the telephone? Are they vulnerable to financial or physical abuse? Are there firearms in the home to which they have access?
In addition to symptoms of dementia, the following atypical symptoms may be seen in the following conditions:
- In patients with Lewy body dementia, symptoms of well-formed visual hallucinations, REM sleep behavior disorder, typical parkinsonian symptoms, and fluctuating cognition, attention, and alertness.[20]
- In patients with frontotemporal dementia, behavior changes, including disinhibition and apathy, and speech difficulties may be seen.[25]
- In patients with Creutzfeld-Jakob disease, symptoms of myoclonus, visual changes, ataxia, and memory and behavior changes are seen.[26]
- In patients with Huntington disease, symptoms of chorea, irritability, and depression can be present.[14]
- In patients with vascular dementia, deficits can occur in stepwise declines.[27]
- In patients with Parkinson disease dementia, symptoms of parkinsonism characterized by bradykinesia, resting tremor, and muscle rigidity are found. In addition, visual hallucinations and delusions may also be seen, especially in the late stages.[20]
Patients with atypical parkinsonian syndromes also bear mentioning. Multiple system atrophy, progressive supranuclear palsy, and corticobasal syndrome have symptoms of parkinsonism in addition to other characteristic findings. Multiple system atrophy has symptoms of autonomic failure and cerebellar ataxia. Progressive supranuclear palsy has symptoms of frequent falls (often backward) and vertical supranuclear gaze palsy. Corticobasal syndrome has progressive asymmetric muscle rigidity and alien limb phenomenon.[28]
The physical exam should be comprehensive, including a complete neurological exam including gait analysis.
Evaluation
The definitive diagnosis of the type of dementia can only be made at autopsy. A probable diagnosis can often be made using clinical history predominantly, sometimes aided by brain imaging and additional laboratory evaluation. Excluding treatable causes of cognitive impairment is also important to include in the initial evaluation.
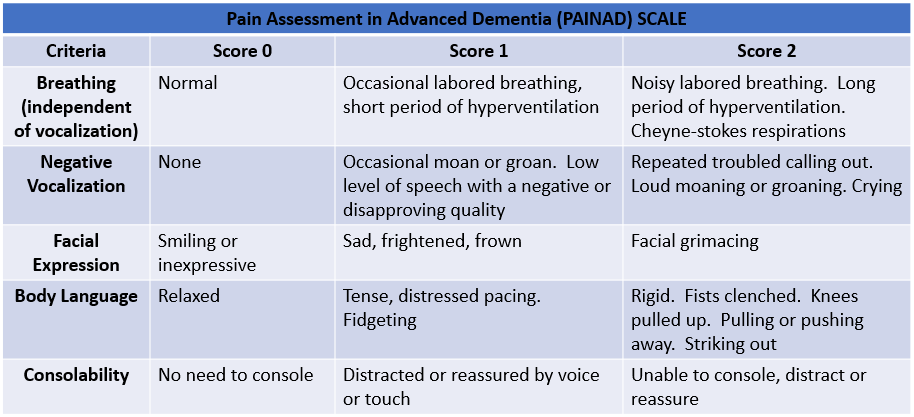
All domains of cognition must be assessed. There are multiple cognitive evaluation tools available for use in a clinical setting, including the Mini-mental status examination (MMSE), Montreal Cognitive Assessment (MoCA), Saint Louis University Mental Status (SLUMS), Addenbrooke's Cognitive Examination–Revised (ACE-R), the modified mini-mental state examination, Mini-Cog, and Rowland Universal Dementia Assessment Scale (RUDAS). See Table. Pain Assessment in Advanced Dementia (PAINAD) Scale.
Each tool has different advantages; for example, the MoCA takes approximately 10 minutes and is better suited for detecting mild cognitive impairment in addition to major neurocognitive disorder. In contrast, the Mini-Cog takes approximately 3 minutes to administer and is predominantly used to screen for major neurocognitive disorder. The RUDAS is often used for use cross-cultural evaluations and can be administered in conjunction with an interpreter if needed. None of these cognitive evaluations alone can diagnose major neurocognitive disorder, as a decline in function of daily tasks is also needed to meet the diagnostic criteria. These studies can be repeated over time to document the progression of decline. They can give an idea of the severity of the deficit along with specific cognitive domains that are affected. Specialized, more in-depth neuropsychological testing can provide even further diagnostic information and help differentiate subtle differences or hard-to-diagnose cases.
Laboratory tests to check in all patients during the evaluation of dementia include complete blood count, urinalysis, metabolic panel, Vitamin B12, folic acid, thyroid function tests, and serological tests for syphilis and HIV. Under certain circumstances, it may be appropriate to check erythrocyte sedimentation rate, lumbar puncture, heavy metal screen, ceruloplasmin levels, Lyme disease titer, or serum protein electrophoresis.
Brain imaging is sometimes ordered, particularly if the age of onset is relatively early, atypical or rapidly progressing symptoms are present, or there is diagnostic uncertainty. A brain MRI without contrast is often the initial test ordered. It is valuable for evaluating signs of vascular or ischemic disease, as well as localized regions or global atrophy that may be seen. A DaTscan uses a radiotracer to highlight dopamine transporter proteins in a SPECT scan on the presynaptic dopaminergic neurons. This scan can aid in differentiating pathologies that involve loss of the striatal dopaminergic pathway, including Parkinson disease, multiple system atrophy, progressive nuclear palsy, cortical-basal degeneration, and Lewy body dementia from other syndromes.[29]
Often reserved for academic settings, functional brain imaging with PET, SPECT, and fMRI can help in the early diagnosis and monitoring of patients with dementia, especially AD. These can also help differentiate the etiology of dementia. These are expensive and routine use in clinical practice is not indicated.[30] There are new CSF and blood tests under research to help identify Alzheimer disease and evaluate concentrations of amyloid and phosphorylated tau proteins as well as markers of neurodegeneration, including neurofilament light chain and glial fibrillar acidic proteins. These tests are not yet ready for regular clinical use.[31]
Treatment / Management
FDA-approved medications to improve cognitive function include cholinesterase inhibitors and memantine. Cholinesterase inhibitors include donepezil, galantamine, and rivastigmine. Cholinesterase inhibitors prevent the breakdown of acetylcholine and aim to slow or delay the worsening of symptoms. Memantine is an NMDA antagonist and decreases the activity of glutamine. Donepezil is approved for all stages of Alzheimer disease, rivastigmine is approved for all stages of Alzheimer disease in its patch form, and mild to moderate stages with oral formulations. Galantamine is approved for mild to moderate stages, and memantine for moderate to severe stages.[32] Acetylcholinesterase inhibitors lead to a variable response among patients, with not all patients showing benefit. There are possible contraindications to their use and significant side effects, including the potential for cardiovascular complications, peptic ulcer disease, and weight loss. Memantine may have neuroprotective benefits as it serves as an uncompetitive antagonist of the NMDA receptor and can prevent neurotoxic and excessive influx of calcium to the neuron.[33] The benefits seen with the use of both acetylcholinesterase inhibitors and memantine are often modest, and many patients and providers choose to forgo pharmacologic treatment.
Aducanumab is a recombinant monoclonal antibody directed against amyloid beta that was recently approved by the FDA for the treatment of mild Alzheimer disease. Its approval remains highly controversial. The drug is costly and does not have clear, proven clinical benefits. It was approved based on positive clinical results seen in only one of the two phase III trials, as well as aducanumab's effect on a surrogate endpoint (reducing amyloid beta plaques in the brain), which has not been proven to be clinically significant.[34]
Lifestyle modifications to optimize cognitive function include optimizing sleep, eating an anti-inflammatory diet, adequate exercise, treating hearing or vision loss, minimizing stress, and maintaining normal blood sugar, cholesterol, and blood pressure levels.[35]
Behavioral symptoms include irritability, anxiety, and depression. Antidepressants and sometimes antipsychotics can help with these symptoms. In addition, non-drug approaches like supportive care, memory training, physical exercise programs, and mental and social stimulation must be employed in symptom control.
Patients and their families should be counseled about the disease and its consequences. They should be provided with all the necessary information in regard to what to expect and how to react to it. Patients and their families should also be encouraged to seek social service consultations and to register with support groups and societies. Coaching caregivers on skills such as redirection and reassurance as opposed to repeated correction of patients confused due to dementia can avoid or de-escalate possible behavioral symptoms. Driving restrictions may have to be imposed.
Differential Diagnosis
- Delirium
- Depression
- Drug use
- Normal age-associated memory changes
- Mild cognitive impairment
- Stress
- Structural brain abnormalities like subdural hematoma, brain tumor, and normal pressure hydrocephalus
- Infections like HIV and neurosyphilis
- Thiamine deficiency
- Vitamin B12 deficiency
- Folic acid deficiency
- Thyroid disorders
- Metabolic abnormalities and derangements
- Medication-induced
- Vitamin E deficiency
Prognosis
The prognosis of dementia is poor. Dementia is often a progressive condition with no cure or treatment. The 1-year mortality rate was 30 to 40%, while the 5-year mortality rate was 60 to 65%. Men had a higher risk than women. Mortality rates among admitted patients with dementia were higher than those with cardiovascular diseases.[36]
Complications
Dementia can affect many body systems and can lead to the following complications:[37]
- Inadequate nutrition
- Pneumonia
- Inability to perform self-care tasks
- Personal safety challenges
- Fractures due to falls
- Hallucinations and delusions
- Apathy
- Agitation
- Dysphagia
- Death
- Depression
- Incontinence
- Personality changes
- Infections
Deterrence and Patient Education
The diagnosis of dementia can be stressful and overwhelming for patients and their families. Patient and caregiver education is a vital part of the clinical management of patients with dementia. Counseling must be given about regular clinic visits, medication compliance, a healthy diet, exercise, and sleep hygiene. Safety concerns become increasingly important as the disease progresses. Special attention should be paid to potential safety concerns, including when to retire from driving, the risk of wandering or getting lost, risks of fire or cooking mishaps, or lack of ability to prepare food for oneself. Patients with dementia often have a lack of insight into their own limitations. Caregivers do better redirecting or reassuring patients rather than trying to correct them. Support groups can help with the reduction of issues like anxiety, frustration, anger, loneliness, and depression. The patients and caregivers should be counseled about the diagnosis and the prognosis. Creating an individualized care plan can empower the patient.
Enhancing Healthcare Team Outcomes
Dementia is a common condition, and its prevalence is expected to increase with time. It is caused by various underlying etiologies and disease states, and each of them may present differently. An interprofessional approach is recommended when managing patients with dementia. Interventions, including care coordination and interprofessional communication, can help reduce hospitalization and decrease emergency department visits.
Physicians must coordinate with other healthcare workers, including other physicians, pharmacists, social workers, and nurses, when managing patients with dementia. Any medication change must be carefully coordinated with all physicians involved in the patient's care. Pharmacists can help with counseling about medication side effects and compliance, and attention should be paid to over-the-counter medications and supplements as much as prescribed medications. Safety in the current living situation must be reviewed during every clinic visit, and social workers may be consulted to assess the safety and adequacy of the living situation and caregiver support. Involving family members and caregivers is an important aspect of the care of patients with dementia. They must be encouraged to accompany the patient during clinic visits to provide an accurate clinical history and to reiterate and enact the plan at home.