Continuing Education Activity
Dengue is a mosquito-transmitted virus, and dengue fever is the leading cause of arthropod-borne viral disease worldwide, posing a significant global health concern. This disease is also known by various monikers, such as breakbone or 7-day fever, and is characterized by intense muscle spasms, joint pain, and high fever, reflecting both the severity and the duration of symptoms. Most dengue virus cases are asymptomatic, yet severe illness and mortality can occur, especially in regions where female Aedes mosquitoes—Aedes aegypti and Aedes albopictus—primarily transmit the virus. Dengue fever, with over 100 million cases annually and 20 to 25,000 deaths, presents a substantial public health challenge, marked by epidemics across different regions globally. Diagnosis usually entails identifying virus antigens using diverse laboratory techniques.
This activity explores the epidemiology of dengue fever, highlighting the increasing incidence observed in tropical and subtropical regions over recent decades, with some areas becoming endemic. This activity also analyzes the complexities of dengue hemorrhagic fever—a severe complication occurring in individuals previously infected with a dengue virus subspecies and subsequently infected with another. In addition, this activity aids clinicians in understanding the etiology, clinical presentation, diagnostic approaches, and management strategies for both dengue fever and dengue hemorrhagic fever, essential for effectively evaluating and addressing this global health threat and providing care to affected individuals.
Objectives:
Identify the clinical manifestations and symptoms of dengue fever.
Screen patients for dengue fever based on presenting symptoms, travel history, and exposure risk.
Apply evidence-based treatment protocols for managing dengue fever, including fluid repletion and symptom management.
Collaborate with interprofessional healthcare providers, including infectious disease specialists and public health authorities, to manage dengue virus outbreaks and improve patient outcomes.
Introduction
Dengue is a mosquito-transmitted virus and is the leading cause of arthropod-borne viral disease worldwide, posing a significant global health concern. This disease is also known by various monikers, such as breakbone or 7-day fever, and is characterized by intense muscle spasms, joint pain, and high fever, reflecting both the severity and the duration of symptoms. Although most dengue fever cases are asymptomatic, severe illness and mortality can occur. Aedes mosquitoes, primarily including the female vectors Aedes aegypti and A albopictus, transmit the virus and are common in tropical and subtropical parts of the world.
The incidence of dengue fever has increased dramatically over the past few decades, and the infection is now endemic in some parts of the world, possibly due to increased global travel. Dengue fever poses a significant public health challenge, with over 100 million cases annually and 20 to 25,000 deaths, marked by epidemics across different regions globally. After infection with a subspecies known as dengue hemorrhagic fever (DHF), some individuals previously infected with one subspecies of the dengue virus (DENV) develop severe capillary permeability and bleeding.[1][2][3] Although the symptoms and signs overlap with several viral prodromes, the identifying features are discussed in the next sections.
Etiology
Dengue fever is caused by any of the 4 distinct serotypes (DENV-1 to DENV-4) of single-stranded RNA viruses belonging to the genus Flavivirus. Infection by one serotype confers lifelong immunity to that serotype but not to others.[4][5][6]
Epidemiology
Dengue fever is the fastest-spreading mosquito-borne viral disease worldwide, affecting over 100 million people annually. This disease also leads to 20 to 25,000 deaths, primarily among children, and is prevalent in more than 100 countries. Epidemics occur yearly in the Americas, Asia, Africa, and Australia.
The dengue virus is maintained by the following 2 transmission cycles:
- Mosquitoes carry the virus from a nonhuman primate to another nonhuman primate
- Mosquitoes transmit the virus from human to human
The human-mosquito cycle primarily occurs in urban environments. Whether the virus transmits from affected humans to mosquitoes depends on the viral load of the mosquitoes' blood meal. The primary vectors of the disease are female mosquitoes of the species Aedes aegypti and Aedes albopictus. Although A aegypti is associated with most infections, the geographic range of A albopictus is expanding. A albopictus, being more cold-tolerant, exhibits aggressive feeding behavior but does so less frequently, which may contribute to its increasing numbers. These mosquito species typically inhabit indoor environments and are active during the day. Modes of transmission include perinatal transmission, blood transfusions, breast milk, and organ transplantation.
Between 1990 and 2010, the mean age of patients was 27.2, which has increased to 34 since 2010. The dengue viral serotype causing disease outbreaks has varied over time, along with the occurrence of severe dengue fever.[7][8] Transmission of the dengue virus generally follows 2 patterns—epidemic dengue and hyperendemic dengue.
Epidemic dengue occurs when a single strain of dengue virus (DENV) is responsible for introduction and transmission, and such epidemics were more common before World War II. During epidemics, all age groups are affected, but the incidence of DHF is relatively low. Hyperendemicity, on the other hand, refers to the co-circulation of various serotypes of DENV in a community linked to periodic outbreaks.[9] In hyperendemic areas, children are affected more than adults, and the incidence of DHF is relatively higher.
Pathophysiology
Belonging to the Flaviviridae family, the dengue virus is a 50-nm virion comprising 3 structural and 7 nonstructural proteins, a lipid envelope, and a 10.7-kb-capped positive-sense single strand of RNA. Infections are asymptomatic in up to 75% of affected individuals. The disease spectrum ranges from self-limiting dengue fever to severe hemorrhage and shock. A fraction of infections, between 0.5% and 5%, develop into severe dengue. Without proper treatment, fatality rates may exceed 20%, particularly among children. The typical incubation period for the disease is 4 to 7 days, with symptoms lasting from 3 to 10 days. Symptoms appearing more than 2 weeks after exposure are unlikely to be attributed to dengue fever.
The consequences of a mosquito bite injecting the dengue virus into the skin remain unclear. Skin macrophages and dendritic cells are believed to be the initial targets. These infected cells are thought to migrate to the lymph nodes and disseminate through the lymphatic system to other organs. Viremia, the presence of the virus in the bloodstream, may occur for 24 to 48 hours before the onset of symptoms.
The presentation of dengue fever, whether asymptomatic, typical, or severe, is influenced by a complex interplay of host and viral factors. Severe dengue fever, characterized by heightened microvascular permeability and shock syndrome, is often associated with infection by a second dengue virus serotype and the patient's immune response. However, severe cases of dengue fever can also arise from infection by a single serotype. Interestingly, microvascular permeability tends to escalate as viral titers decrease.
History and Physical
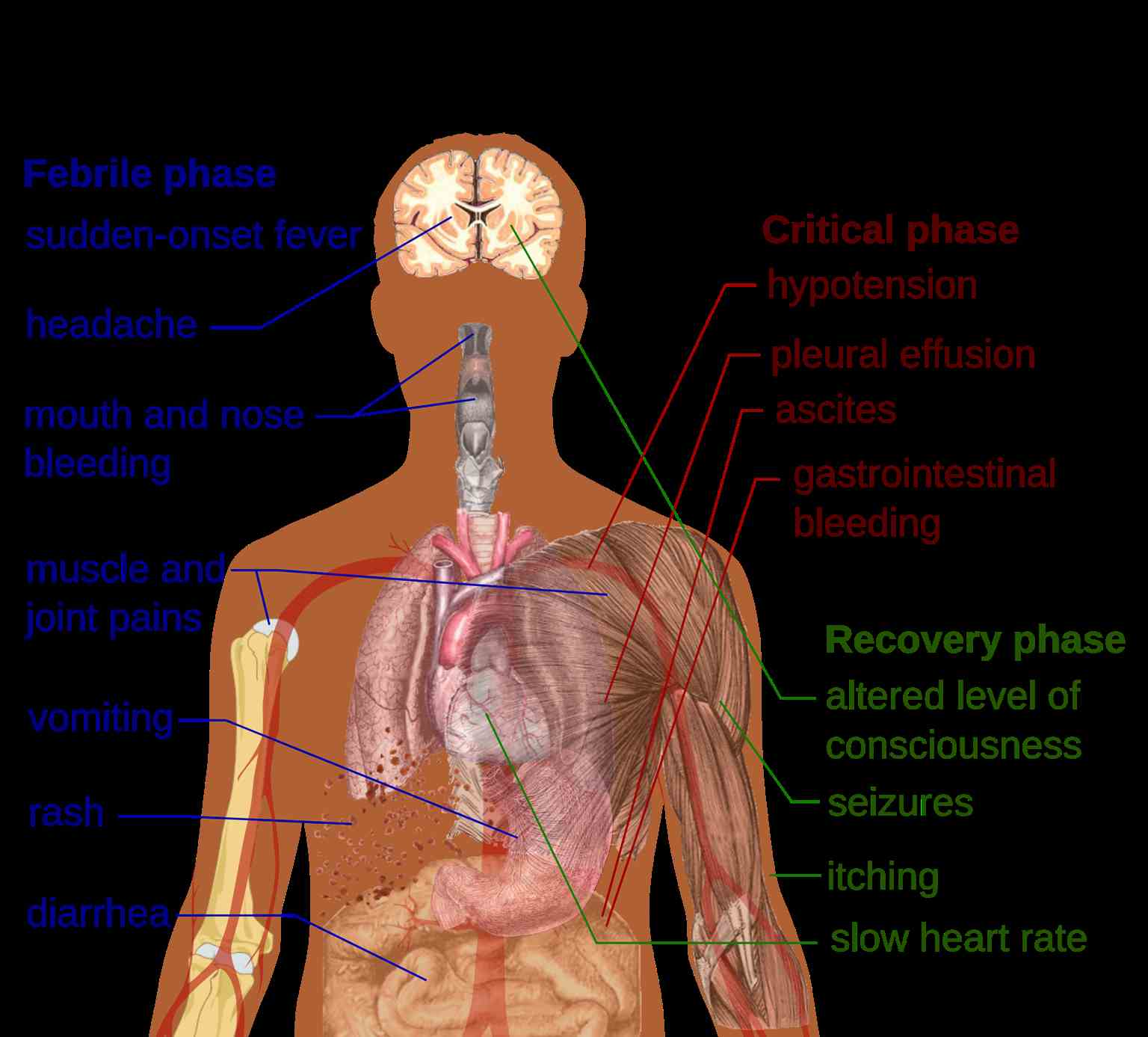
The 3 phases of dengue fever include febrile, critical, and recovery stages (see Image. Primary Symptoms of Dengue Fever).
The febrile phase: During the febrile phase, individuals typically experience a sudden onset of high-grade fever, reaching approximately 40 °C, which usually lasts for 2 to 7 days. Approximately 6% of cases may exhibit saddleback or biphasic fever, particularly in patients with DHF and severe dengue fever. The fever usually persists for at least 24 hours, followed by a subsequent spike lasting at least 1 more day.[10] Associated symptoms during this phase include facial flushing, skin erythema, myalgias, arthralgias, headache, sore throat, conjunctival injection, anorexia, nausea, and vomiting. Skin erythema manifests as a general blanchable macular rash within 1 to 2 days of fever onset and again on the last day. Alternatively, within 24 hours, a secondary maculopapular rash may develop.
The critical phase: During the critical phase, defervescence marks a period when the temperature typically decreases to approximately 37.5 to 38 °C or lower, occurring between days 3 and 7. This phase is associated with heightened capillary permeability and typically lasts for 1 to 2 days. Before the critical phase, there is often a rapid decline in platelet count, accompanied by increased hematocrit levels. Leukopenia may also occur up to 24 hours before the platelet count drops and warning signs emerge. If left untreated, the critical phase can progress to shock, organ dysfunction, disseminated intravascular coagulation, or hemorrhage.
The recovery phase: The recovery phase involves the gradual reabsorption of extravascular fluid over 2 to 3 days. During this period, patients often exhibit bradycardia.
Expanded dengue virus syndrome refers to unusual or atypical manifestations seen in patients with involvement of various organs such as neurological, hepatic, and renal. This syndrome can be associated with profound shock. Neurological manifestations may include febrile seizures in young children, encephalitis, aseptic meningitis, and intracranial bleeding. Gastrointestinal involvement might present as hepatitis, liver failure, pancreatitis, or acalculous cholecystitis. In addition, this syndrome can manifest as myocarditis, pericarditis, acute respiratory distress syndrome, acute kidney injury, or hemolytic uremic syndrome.
Evaluation
Common laboratory findings include thrombocytopenia, leukopenia, and elevated levels of aspartate aminotransferase. The disease is classified as either dengue or severe dengue.[11][12][13]
- Probable dengue: The patient lives in or has traveled to a Dengue-endemic area. Symptoms include fever and 2 of the following: nausea, vomiting, rash, myalgias, arthralgias, rash, positive tourniquet test, or leukopenia.
- Warning signs of dengue: Dengue symptoms include abdominal pain, persistent vomiting, clinical fluid accumulation such as ascites or pleural effusion, mucosal bleeding, lethargy, liver enlargement greater than 2 cm, increase in hematocrit, and thrombocytopenia.
- Severe dengue: Severe dengue is characterized by dengue fever accompanied by severe plasma leakage, hemorrhage, impaired consciousness, myocardial dysfunction, pulmonary dysfunction, and organ dysfunction, including transaminitis greater than 1000 IU/L.
- Dengue shock syndrome clinical warnings: Symptoms include rapidly rising hematocrit, intense abdominal pain, persistent vomiting, and narrowed or absent blood pressure.
The virus antigen can be detected using enzyme-linked immunosorbent assay (ELISA) test, polymerase chain reaction (PCR), or by isolating the virus from body fluids. Serology typically shows a significant increase in immunoglobulins. A confirmed diagnosis is established through culture, antigen detection, PCR, or serologic testing. Notably, it is crucial to evaluate pregnant patients with dengue carefully, as the symptoms can resemble those of preeclampsia.
Treatment / Management
The treatment approach for dengue fever varies depending on the patient's illness phase. Patients without warning signs can typically be treated as outpatients with acetaminophen and sufficient oral fluids. In addition, educating patients about the warning signs and advising them to seek immediate medical attention if any of these signs occur is important.
Patients presenting with warning signs of the disease, severe dengue fever, or having risk factors such as age, pregnancy status, diabetes mellitus, or those who are living alone should be evaluated for hospitalization. Individuals displaying warning signs can be started on intravenous (IV) crystalloids, with the fluid rate adjusted based on the patient's response. Patients in shock and not responding to initial crystalloid boluses may require colloids.
Blood transfusion is indicated in cases of severe or suspected bleeding when the patient remains unstable despite adequate fluid resuscitation and hematocrit falls. Platelet transfusion may be necessary if the platelet count drops below 20,000 cells per microliter and there is a high risk of bleeding. Notably, it is essential to avoid administering aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), and other anticoagulants. No antiviral medications are recommended, and no laboratory tests can reliably predict the progression to severe disease.
Differential Diagnosis
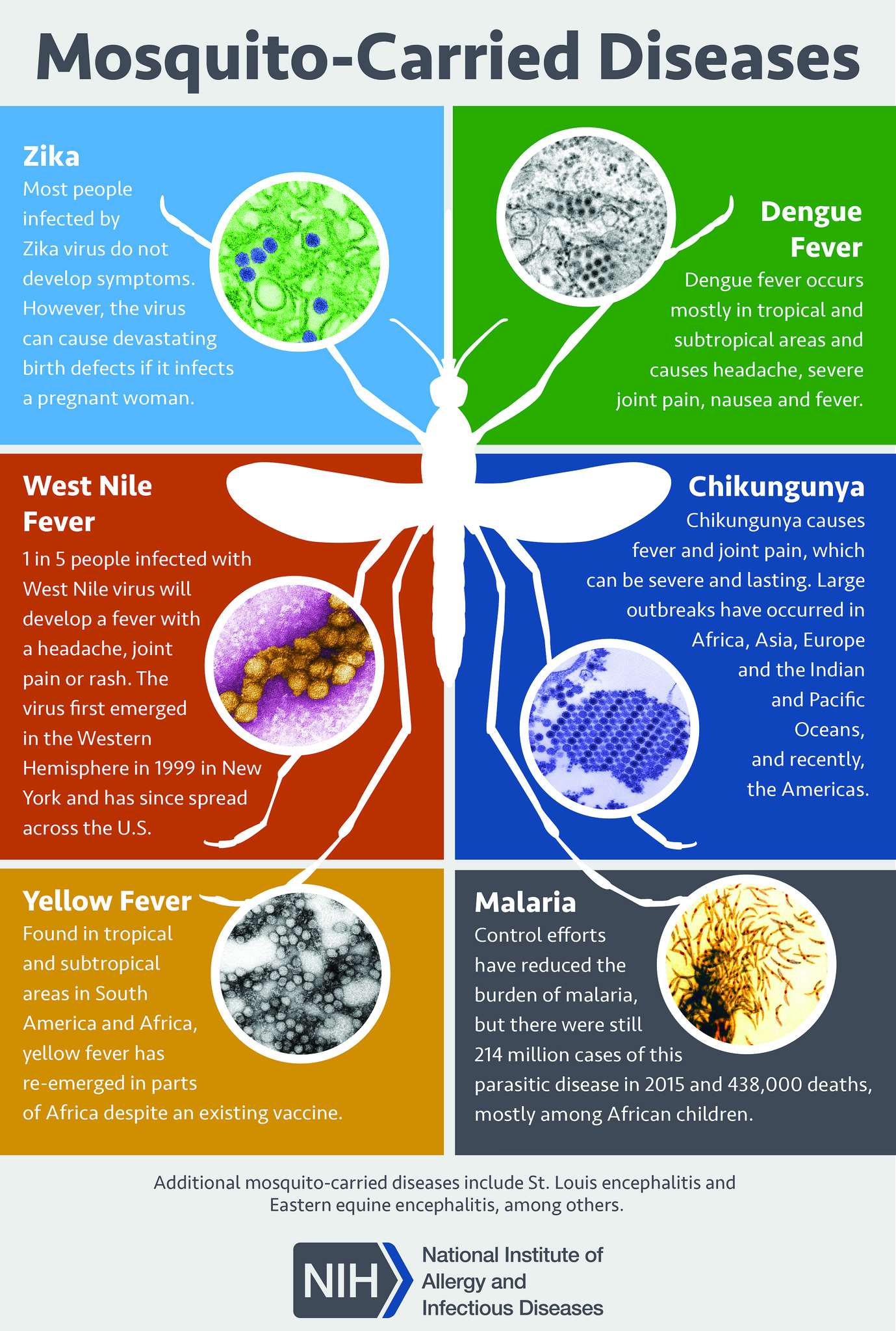
The clinical diagnosis of dengue fever can be challenging as many other illnesses can present similarly early in the disease course. Other differential diagnoses include measles, influenza, and mosquito-vector diseases such as Zika virus disease, West Nile infection, chikungunya, malaria, and yellow fever (see Image. Mosquito-Borne Diseases).
Obtaining a detailed history of immunizations, travel, and exposures is crucial for the diagnosis of dengue fever. Rapid laboratory identification of the dengue virus involves NS1 antigen detection and serological tests. Serological tests are only helpful after several days of infection and may yield false positives due to other Flavivirus infections, such as yellow fever or Zika virus.
Prognosis
Untreated severe dengue fever may have a mortality rate of 10% to 20%. However, with appropriate supportive care, the mortality rate can be reduced to approximately 1%.
Complications
Complications of dengue fever may include liver injury, cardiomyopathy, pneumonia, orchitis, oophoritis, seizures, encephalopathy, and encephalitis.
Postoperative and Rehabilitation Care
Patients should be encouraged to drink plenty of fluids. The return of appetite in a patient is a sign that the infection is subsiding.
Consultations
Consulting an infectious disease specialist is recommended, as many clinicians have limited experience managing this infection. The Centers for Disease Control and Prevention (CDC) also provides a hotline offering treatment advice.
Deterrence and Patient Education
The only way to avoid contracting dengue virus is to prevent mosquito bites and avoid endemic areas.
Preventative Measures
- Using bed nets from daytime onward.
- Utilizing insecticide-treated materials such as window curtains.
- Applying mosquito-repellant creams containing DEET, IR3535, or icaridin.
- Using mosquito-repellant coils.
- Developing the habit of wearing long-sleeved shirts and pants.[14]
Biological Control
- Fish: Introducing viviparous species of fish, such as Poecilia reticulata, into confined water bodies such as large water tanks or open freshwater wells, and utilizing native larvicidal fish.
- Predatory copepods: Implementing small freshwater crustaceans as effective predators, particularly in specific container habitats.
- Endosymbiotic control: Utilizing mosquitoes infected with Wolbachia, an intracellular parasite, as they demonstrate reduced susceptibility to DENV infection compared to wild-type mosquitoes A aegypti.[15]
Chemical Control
- Using larvicidal in big breeding containers.
- Applying insecticide sprays via space sprays, which can be administered as thermal fogs or cold aerosols.
- Using oil-based formulations, as they inhibit evaporation
- Using a few common insecticides such as organophosphorus compounds (fenitrothion and malathion) and pyrethroids (bioresmethrin and cypermethrin).
Environmental Measures
- Identifying and eliminating the breeding areas of mosquitoes and pests.
- Maintaining the rooftops and sunshades properly.
- Covering stored water in buckets, pots, and other vessels appropriately.
Health Education
Educating individuals about the dengue virus is crucial for effective public health interventions. Utilizing audiovisual and mass awareness campaigns can serve as initial steps in disseminating knowledge about the virus, which can be implemented at both individual and population levels.
Vaccination
CYD-TDV, the first licensed live recombinant tetravalent dengue vaccine, is approved for use in endemic areas across 20 countries.[16]
Enhancing Healthcare Team Outcomes
Diagnosing and managing dengue fever involve a multidisciplinary team of healthcare professionals comprising an infectious disease expert, a CDC consultant, an emergency department clinician, and an internist. Treatment primarily focuses on supportive care, including fluid repletion, acetaminophen for fever management, and blood transfusion if hemorrhage occurs. A confirmed diagnosis is established through various methods such as culture, antigen detection, polymerase chain reaction, or serologic testing.
Laboratory tests cannot reliably predict the progression to severe disease. Primary care clinicians and nurse practitioners play a crucial role in educating travelers on preventing mosquito bites and adopting preventive measures such as covering their exposed skin, using bed nets, mosquito repellents, and indoor insecticides, as well as eliminating mosquito breeding grounds such as standing water. While the prognosis for untreated dengue fever is poor, most patients can survive with supportive care, although some may experience residual multisystem organ damage.[17][18]