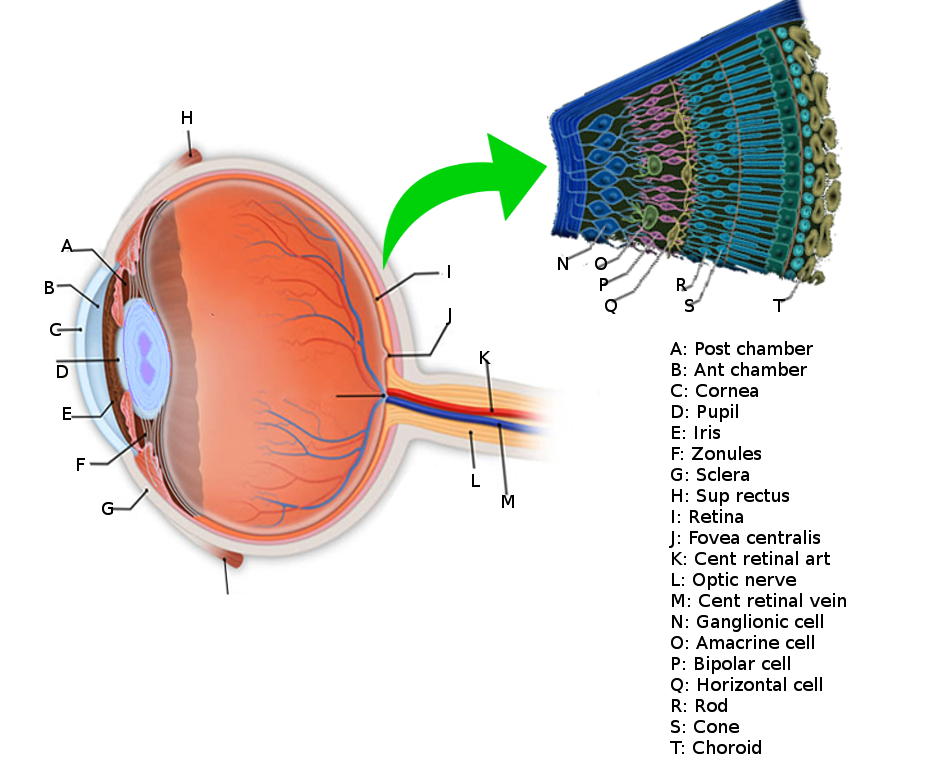
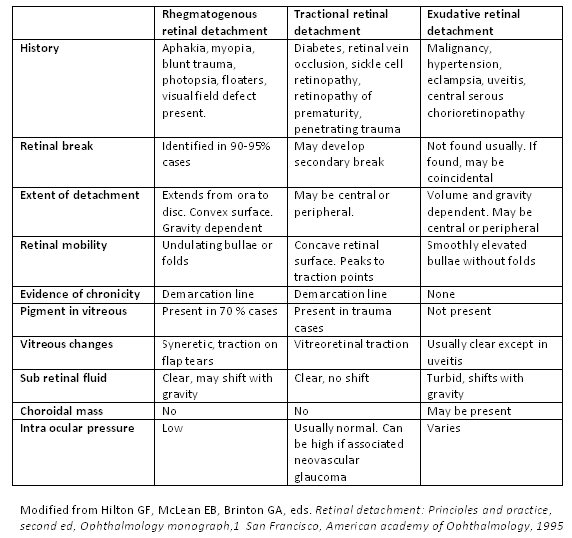
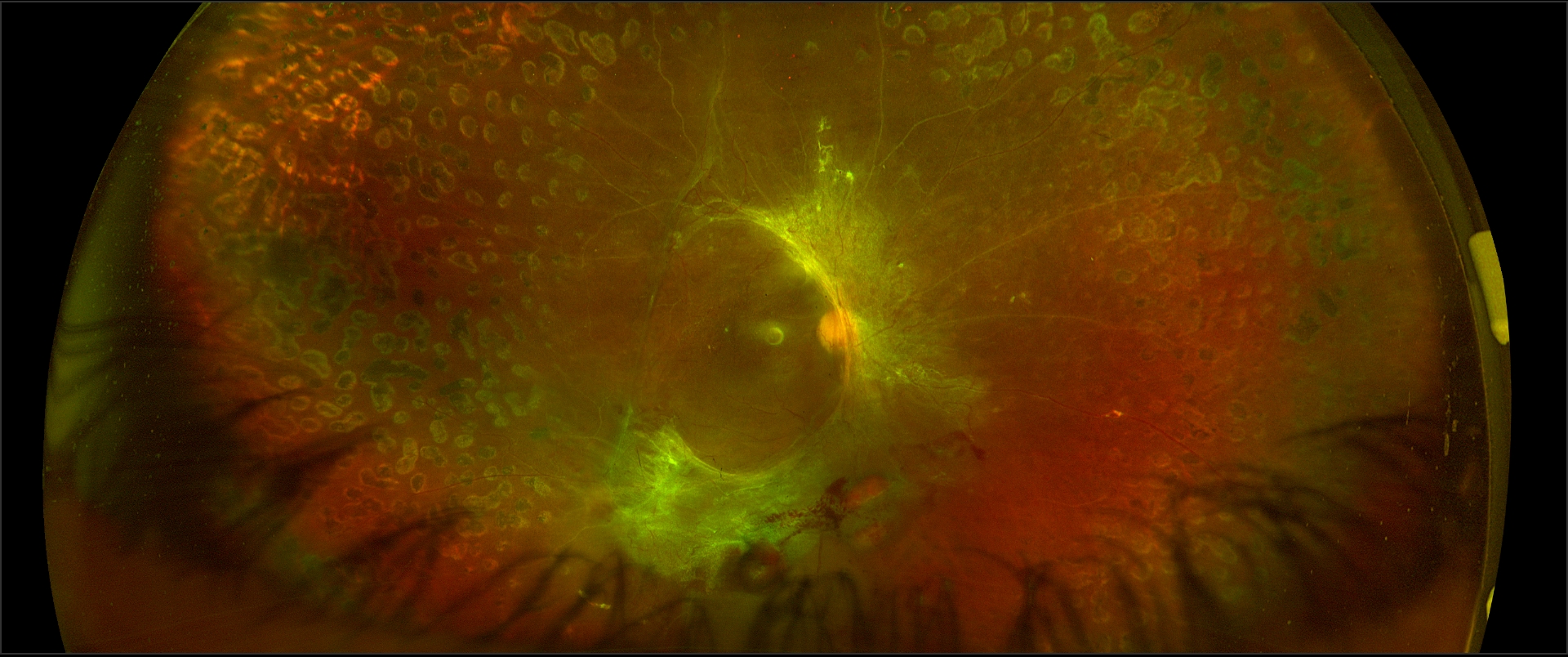
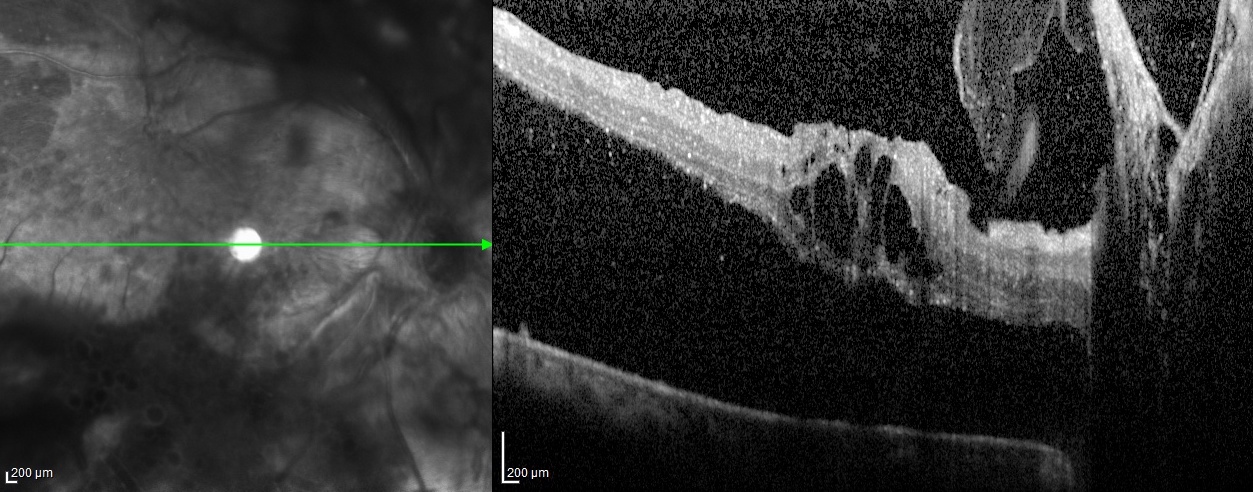
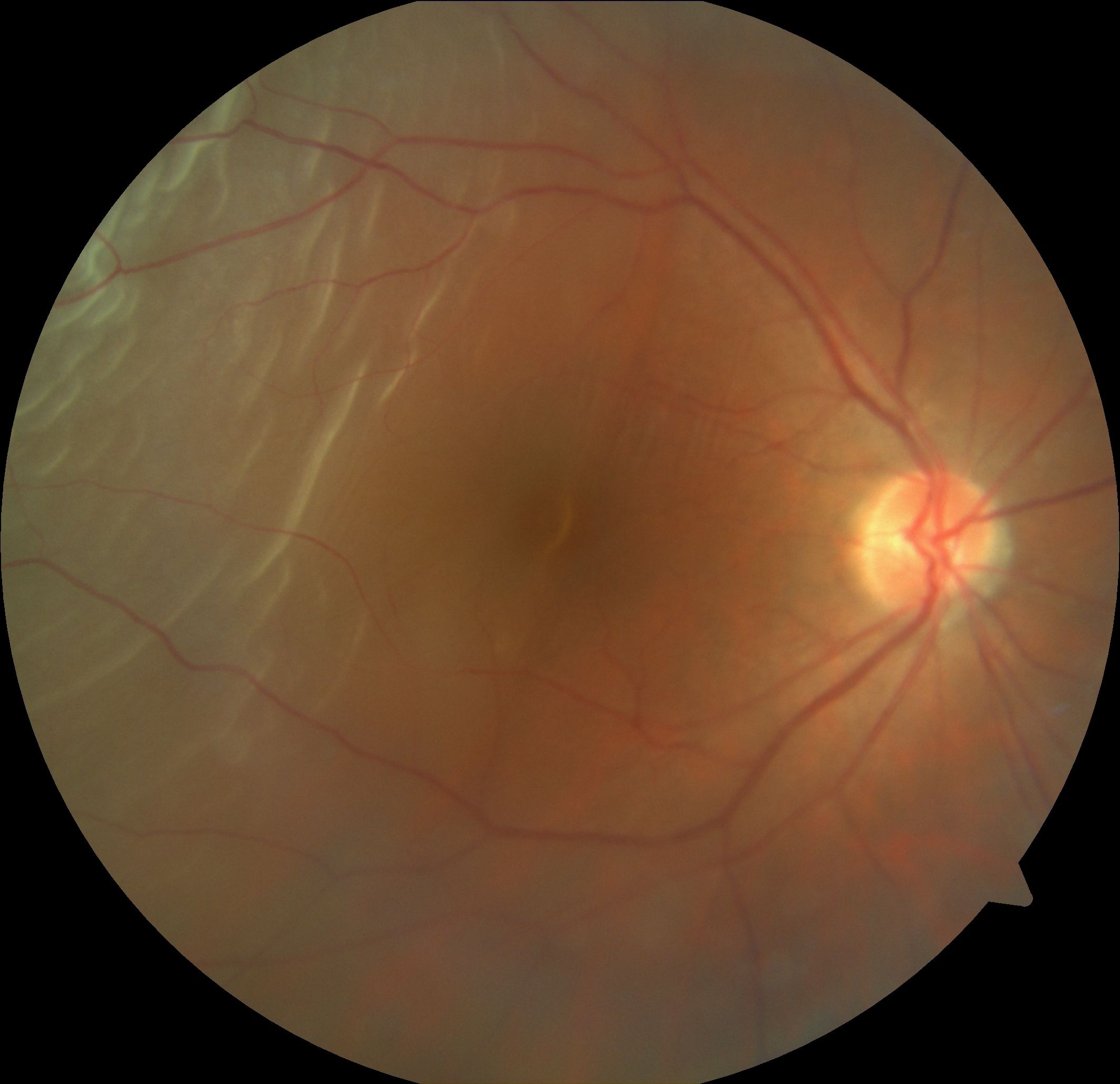
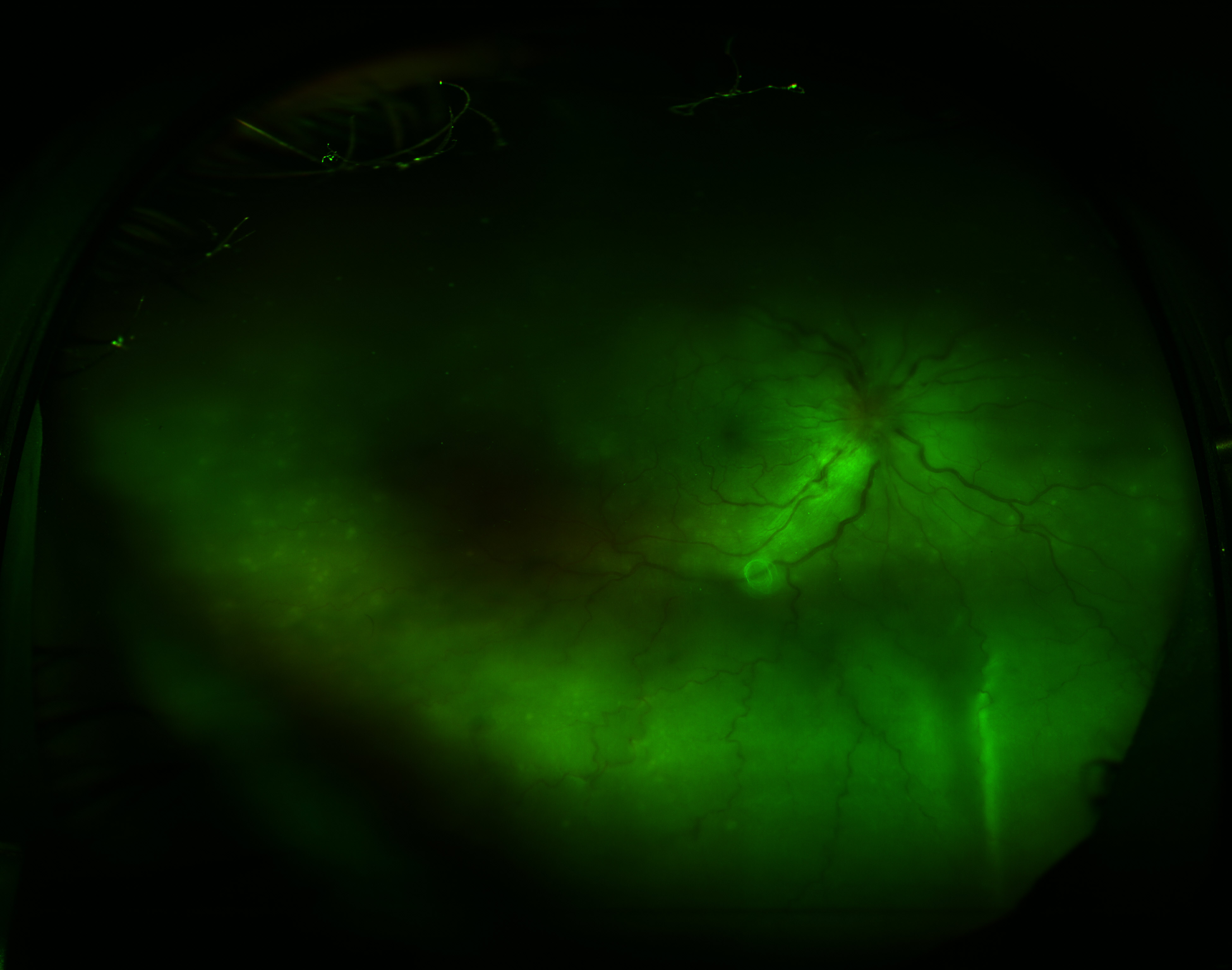
[4]
Rasouli M, Steed SM, Tennant MT, Rudnisky CJ, Hinz BJ, Greve MD, Somani R. The 1-year incidence of rhegmatogenous retinal detachment post 23-gauge pars plana vitrectomy. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2012 Jun:47(3):262-3. doi: 10.1016/j.jcjo.2012.03.015. Epub 2012 May 10
[PubMed PMID: 22687303]
[5]
Gupta OP, Benson WE. The risk of fellow eyes in patients with rhegmatogenous retinal detachment. Current opinion in ophthalmology. 2005 Jun:16(3):175-8
[PubMed PMID: 15870575]
Level 3 (low-level) evidence
[6]
Johnston T, Chandra A, Hewitt AW. Current Understanding of the Genetic Architecture of Rhegmatogenous Retinal Detachment. Ophthalmic genetics. 2016 Jun:37(2):121-9. doi: 10.3109/13816810.2015.1033557. Epub 2016 Jan 12
[PubMed PMID: 26757352]
Level 3 (low-level) evidence
[9]
Shukla M, Ahuja OP. A possible relationship between lattice and snail track degenerations of the retina. American journal of ophthalmology. 1981 Oct:92(4):482-5
[PubMed PMID: 7294110]
[10]
Byer NE. Lattice degeneration of the retina. Survey of ophthalmology. 1979 Jan-Feb:23(4):213-48
[PubMed PMID: 424991]
Level 3 (low-level) evidence
[11]
Tripathy K, Chawla R, Sharma YR, Venkatesh P, Sagar P, Vohra R, Singh HI, Kumawat B, Bypareddy R. Prophylactic laser photocoagulation of fundal coloboma: does it really help? Acta ophthalmologica. 2016 Dec:94(8):e809-e810. doi: 10.1111/aos.12975. Epub 2016 Jan 29
[PubMed PMID: 26821601]
[13]
Roy S, Madan R, Gogia A, Tripathy K, Sharma D, Julka PK, Rath GK. Short course palliative radiotherapy in the management of choroidal metastasis: An effective technique since ages. Journal of the Egyptian National Cancer Institute. 2016 Mar:28(1):49-53. doi: 10.1016/j.jnci.2015.07.003. Epub 2015 Jul 31
[PubMed PMID: 26239538]
[15]
Paulbuddhe V, Addya S, Gurnani B, Singh D, Tripathy K, Chawla R. Sympathetic Ophthalmia: Where Do We Currently Stand on Treatment Strategies? Clinical ophthalmology (Auckland, N.Z.). 2021:15():4201-4218. doi: 10.2147/OPTH.S289688. Epub 2021 Oct 20
[PubMed PMID: 34707340]
[16]
Chawla R, Kapoor M, Mehta A, Tripathy K, Vohra R, Venkatesh P. Sympathetic Ophthalmia: Experience from a Tertiary Care Center in Northern India. Journal of ophthalmic & vision research. 2018 Oct-Dec:13(4):439-446. doi: 10.4103/jovr.jovr_86_17. Epub
[PubMed PMID: 30479714]
[17]
Tripathy K, Mittal K, Chawla R. Sympathetic ophthalmia following a conjunctival flap procedure for corneal perforation. BMJ case reports. 2016 Mar 14:2016():. doi: 10.1136/bcr-2016-214344. Epub 2016 Mar 14
[PubMed PMID: 26976837]
Level 3 (low-level) evidence
[19]
Venkatesh P, Chawla R, Tripathy K, Singh HI, Bypareddy R. Scleral resection in chronic central serous chorioretinopathy complicated by exudative retinal detachment. Eye and vision (London, England). 2016:3(1):23. doi: 10.1186/s40662-016-0055-5. Epub 2016 Sep 9
[PubMed PMID: 27617266]
[20]
Chawla R, Tripathy K, Meena S, Behera AK. Subretinal Hypopyon in Presumed Tubercular Uveitis: A Report of Two Cases. Middle East African journal of ophthalmology. 2018 Jul-Dec:25(3-4):163-166. doi: 10.4103/meajo.MEAJO_187_17. Epub
[PubMed PMID: 30765956]
Level 3 (low-level) evidence
[21]
Tripathy K, Chawla R. Choroidal tuberculoma. The National medical journal of India. 2016 Mar-Apr:29(2):106
[PubMed PMID: 27586221]
[22]
Tripathy K, Chawla R, Mutha V, Selvan H. Spontaneous suprachoroidal haemorrhage with exudative retinal detachment in pregnancy-induced hypertension. BMJ case reports. 2018 Mar 9:2018():. pii: bcr-2017-223907. doi: 10.1136/bcr-2017-223907. Epub 2018 Mar 9
[PubMed PMID: 29523618]
Level 3 (low-level) evidence
[23]
Tripathy K, Chawla R. Bilateral exudative retinal detachment with choroidopathy in malignant hypertension. The National medical journal of India. 2015 Sep-Oct:28(5):261
[PubMed PMID: 27132968]
[25]
Gupta A, Paulbuddhe VS, Shukla UV, Tripathy K. Exudative Retinitis (Coats Disease). StatPearls. 2022 Jan:():
[PubMed PMID: 32809517]
[26]
Mitry D, Charteris DG, Fleck BW, Campbell H, Singh J. The epidemiology of rhegmatogenous retinal detachment: geographical variation and clinical associations. The British journal of ophthalmology. 2010 Jun:94(6):678-84. doi: 10.1136/bjo.2009.157727. Epub 2009 Jun 9
[PubMed PMID: 19515646]
[27]
Mitry D, Tuft S, McLeod D, Charteris DG. Laterality and gender imbalances in retinal detachment. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2011 Jul:249(7):1109-10. doi: 10.1007/s00417-010-1529-0. Epub 2010 Oct 1
[PubMed PMID: 20886223]
[28]
Chandra A, Banerjee P, Davis D, Charteris D. Ethnic variation in rhegmatogenous retinal detachments. Eye (London, England). 2015 Jun:29(6):803-7. doi: 10.1038/eye.2015.43. Epub 2015 Mar 27
[PubMed PMID: 25853394]
[29]
Rosman M, Wong TY, Ong SG, Ang CL. Retinal detachment in Chinese, Malay and Indian residents in Singapore: a comparative study on risk factors, clinical presentation and surgical outcomes. International ophthalmology. 2001:24(2):101-6
[PubMed PMID: 12201344]
Level 2 (mid-level) evidence
[30]
Sultan ZN, Agorogiannis EI, Iannetta D, Steel D, Sandinha T. Rhegmatogenous retinal detachment: a review of current practice in diagnosis and management. BMJ open ophthalmology. 2020:5(1):e000474. doi: 10.1136/bmjophth-2020-000474. Epub 2020 Oct 9
[PubMed PMID: 33083551]
[32]
Iyer SSR, Regan KA, Burnham JM, Chen CJ. Surgical management of diabetic tractional retinal detachments. Survey of ophthalmology. 2019 Nov-Dec:64(6):780-809. doi: 10.1016/j.survophthal.2019.04.008. Epub 2019 May 9
[PubMed PMID: 31077688]
Level 3 (low-level) evidence
[33]
Sisk RA. Intraoperative Drainage of a Bullous Serous Pigment Epithelial Detachment. Ophthalmic surgery, lasers & imaging retina. 2019 Aug 1:50(8):510-513. doi: 10.3928/23258160-20190806-06. Epub
[PubMed PMID: 31415698]
[34]
Hollands H, Johnson D, Brox AC, Almeida D, Simel DL, Sharma S. Acute-onset floaters and flashes: is this patient at risk for retinal detachment? JAMA. 2009 Nov 25:302(20):2243-9. doi: 10.1001/jama.2009.1714. Epub
[PubMed PMID: 19934426]
[35]
Broadway DC. How to test for a relative afferent pupillary defect (RAPD). Community eye health. 2016:29(96):68-69
[PubMed PMID: 28381906]
[37]
D'Amico DJ. Clinical practice. Primary retinal detachment. The New England journal of medicine. 2008 Nov 27:359(22):2346-54. doi: 10.1056/NEJMcp0804591. Epub
[PubMed PMID: 19038880]
[38]
Tran KD, Schwartz SG, Smiddy WE, Flynn HW Jr. The Role of Scleral Depression in Modern Clinical Practice. American journal of ophthalmology. 2018 Nov:195():xviii-xix. doi: 10.1016/j.ajo.2018.08.017. Epub 2018 Sep 26
[PubMed PMID: 30268376]
[39]
Lahham S, Shniter I, Thompson M, Le D, Chadha T, Mailhot T, Kang TL, Chiem A, Tseeng S, Fox JC. Point-of-Care Ultrasonography in the Diagnosis of Retinal Detachment, Vitreous Hemorrhage, and Vitreous Detachment in the Emergency Department. JAMA network open. 2019 Apr 5:2(4):e192162. doi: 10.1001/jamanetworkopen.2019.2162. Epub 2019 Apr 5
[PubMed PMID: 30977855]
[40]
Hallinan JT, Pillay P, Koh LH, Goh KY, Yu WY. Eye Globe Abnormalities on MR and CT in Adults: An Anatomical Approach. Korean journal of radiology. 2016 Sep-Oct:17(5):664-73. doi: 10.3348/kjr.2016.17.5.664. Epub 2016 Aug 23
[PubMed PMID: 27587955]
[41]
Gottlieb M, Holladay D, Peksa GD. Point-of-Care Ocular Ultrasound for the Diagnosis of Retinal Detachment: A Systematic Review and Meta-Analysis. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 2019 Aug:26(8):931-939. doi: 10.1111/acem.13682. Epub 2019 Feb 5
[PubMed PMID: 30636351]
Level 1 (high-level) evidence
[42]
Forte R, Pascotto F, de Crecchio G. Visualization of vitreomacular tractions with en face optical coherence tomography. Eye (London, England). 2007 Nov:21(11):1391-4
[PubMed PMID: 16751756]
[43]
Lincoff H, Gieser R. Finding the retinal hole. Archives of ophthalmology (Chicago, Ill. : 1960). 1971 May:85(5):565-9
[PubMed PMID: 5087597]
[44]
Elhusseiny AM, Yannuzzi NA, Smiddy WE. Cost Analysis of Pneumatic Retinopexy versus Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment. Ophthalmology. Retina. 2019 Nov:3(11):956-961. doi: 10.1016/j.oret.2019.06.003. Epub 2019 Jun 12
[PubMed PMID: 31416765]
[45]
Hoerauf H, Heimann H, Hansen L, Laqua H. [Scleral buckling surgery and pneumatic retinopexy. Techniques, indications and results]. Der Ophthalmologe : Zeitschrift der Deutschen Ophthalmologischen Gesellschaft. 2008 Jan:105(1):7-18. doi: 10.1007/s00347-007-1673-z. Epub
[PubMed PMID: 18210120]
[46]
Ahmadieh H, Moradian S, Faghihi H, Parvaresh MM, Ghanbari H, Mehryar M, Heidari E, Behboudi H, Banaee T, Golestan B, Pseudophakic and Aphakic Retinal Detachment (PARD) Study Group. Anatomic and visual outcomes of scleral buckling versus primary vitrectomy in pseudophakic and aphakic retinal detachment: six-month follow-up results of a single operation--report no. 1. Ophthalmology. 2005 Aug:112(8):1421-9
[PubMed PMID: 15961159]
[47]
Stangos AN, Petropoulos IK, Brozou CG, Kapetanios AD, Whatham A, Pournaras CJ. Pars-plana vitrectomy alone vs vitrectomy with scleral buckling for primary rhegmatogenous pseudophakic retinal detachment. American journal of ophthalmology. 2004 Dec:138(6):952-8
[PubMed PMID: 15629285]
[48]
Ross WH, Lavina A. Pneumatic retinopexy, scleral buckling, and vitrectomy surgery in the management of pseudophakic retinal detachments. Canadian journal of ophthalmology. Journal canadien d'ophtalmologie. 2008 Feb:43(1):65-72. doi: 10.3129/i07-196. Epub
[PubMed PMID: 18204500]
[49]
Schwartz SG, Flynn HW. Pars plana vitrectomy for primary rhegmatogenous retinal detachment. Clinical ophthalmology (Auckland, N.Z.). 2008 Mar:2(1):57-63
[PubMed PMID: 19668388]
[51]
Stewart MW, Browning DJ, Landers MB. Current management of diabetic tractional retinal detachments. Indian journal of ophthalmology. 2018 Dec:66(12):1751-1762. doi: 10.4103/ijo.IJO_1217_18. Epub
[PubMed PMID: 30451175]
[52]
Ip M, Garza-Karren C, Duker JS, Reichel E, Swartz JC, Amirikia A, Puliafito CA. Differentiation of degenerative retinoschisis from retinal detachment using optical coherence tomography. Ophthalmology. 1999 Mar:106(3):600-5
[PubMed PMID: 10080221]
[54]
Hsu YJ, Hsieh YT, Yeh PT, Huang JY, Yang CM. Combined Tractional and Rhegmatogenous Retinal Detachment in Proliferative Diabetic Retinopathy in the Anti-VEGF Era. Journal of ophthalmology. 2014:2014():917375. doi: 10.1155/2014/917375. Epub 2014 Jun 25
[PubMed PMID: 25061523]
[55]
Semidey VA,Al Taisan AA,Schatz P,Taskintuna I,Mura M, Surgical Management of Hemorrhagic Retinal Detachment Secondary to Peripheral Exudative Hemorrhagic Chorioretinopathy. Middle East African journal of ophthalmology. 2021 Jan-Mar;
[PubMed PMID: 34321823]
[56]
Wykoff CC, Smiddy WE, Mathen T, Schwartz SG, Flynn HW Jr, Shi W. Fovea-sparing retinal detachments: time to surgery and visual outcomes. American journal of ophthalmology. 2010 Aug:150(2):205-210.e2. doi: 10.1016/j.ajo.2010.03.002. Epub 2010 Jun 11
[PubMed PMID: 20541738]
[57]
Lee IT, Lampen SIR, Wong TP, Major JC Jr, Wykoff CC. Fovea-sparing rhegmatogenous retinal detachments: impact of clinical factors including time to surgery on visual and anatomic outcomes. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2019 May:257(5):883-889. doi: 10.1007/s00417-018-04236-4. Epub 2019 Jan 11
[PubMed PMID: 30635720]
[59]
Grabowska A, Neffendorf JE, Yorston D, Williamson TH. Urgency of retinal detachment repair: is it time to re-think our priorities? Eye (London, England). 2021 Apr:35(4):1035-1036. doi: 10.1038/s41433-020-01154-w. Epub 2020 Sep 1
[PubMed PMID: 32873942]
[60]
Christensen U, Villumsen J. Prognosis of pseudophakic retinal detachment. Journal of cataract and refractive surgery. 2005 Feb:31(2):354-8
[PubMed PMID: 15767158]
[61]
Sobol WM, Blodi CF, Folk JC, Weingeist TA. Long-term visual outcome in patients with optic nerve pit and serous retinal detachment of the macula. Ophthalmology. 1990 Nov:97(11):1539-42
[PubMed PMID: 2255526]
[62]
Piccolino FC, de la Longrais RR, Ravera G, Eandi CM, Ventre L, Abdollahi A, Manea M. The foveal photoreceptor layer and visual acuity loss in central serous chorioretinopathy. American journal of ophthalmology. 2005 Jan:139(1):87-99
[PubMed PMID: 15652832]
[63]
Idrees S, Sridhar J, Kuriyan AE. Proliferative Vitreoretinopathy: A Review. International ophthalmology clinics. 2019 Winter:59(1):221-240. doi: 10.1097/IIO.0000000000000258. Epub
[PubMed PMID: 30585928]
[64]
Sharma A, Grigoropoulos V, Williamson TH. Management of primary rhegmatogenous retinal detachment with inferior breaks. The British journal of ophthalmology. 2004 Nov:88(11):1372-5
[PubMed PMID: 15489475]
[66]
Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, Ying GS. Posterior Vitreous Detachment, Retinal Breaks, and Lattice Degeneration Preferred Practice Pattern®. Ophthalmology. 2020 Jan:127(1):P146-P181. doi: 10.1016/j.ophtha.2019.09.027. Epub 2019 Sep 25
[PubMed PMID: 31757500]
[67]
Koçak N, Kaya M, Öztürk T, Bolluk V, Kaynak S. Demarcation Laser Photocoagulation for Subclinical Retinal Detachment: Can Progression to Retinal Detachment Be Prevented? Turkish journal of ophthalmology. 2019 Dec 31:49(6):342-346. doi: 10.4274/tjo.galenos.2019.22844. Epub
[PubMed PMID: 31893590]
[68]
Uhumwangho OM,Jalali S, Chorioretinal coloboma in a paediatric population. Eye (London, England). 2014 Jun;
[PubMed PMID: 24675580]
[69]
Fan S, Lin D, Wu R, Wang Y. Efficacy of prophylactic laser retinopexy in acute retinal necrosis: A systematic review and meta-analysis. International ophthalmology. 2022 May:42(5):1651-1660. doi: 10.1007/s10792-021-02131-2. Epub 2022 Mar 21
[PubMed PMID: 35307785]
Level 1 (high-level) evidence
[70]
Bshouti E, Hoban K, Affel E, Bourbonniere M, Line C, Murchison AP. Ocular emergencies in an ophthalmic emergency room. Insight (American Society of Ophthalmic Registered Nurses). 2014 Summer:39(3):18-21
[PubMed PMID: 25195337]
[71]
Eijk ES, Busschbach JJ, Timman R, Monteban HC, Vissers JM, van Meurs JC. What made you wait so long? Delays in presentation of retinal detachment: knowledge is related to an attached macula. Acta ophthalmologica. 2016 Aug:94(5):434-40. doi: 10.1111/aos.13016. Epub 2016 Mar 24
[PubMed PMID: 27008986]
[72]
Quinn SM, Qureshi F, Charles SJ. Assessment of delays in presentation of patients with retinal detachment to a tertiary referral centre. Ophthalmic & physiological optics : the journal of the British College of Ophthalmic Opticians (Optometrists). 2004 Mar:24(2):100-5
[PubMed PMID: 15005674]