Continuing Education Activity
Chronic venous insufficiency (CVI) is a condition that encompasses several pathological changes (eg, lower extremity edema, skin trophic changes, and discomfort) secondary to venous hypertension. Chronic venous insufficiency is a prevalent disease process around the world. Disability associated with chronic venous insufficiency contributes to a diminished quality of life and loss of work productivity. In most cases, the underlying cause is incompetent venous valves. Each year, approximately 150,000 new patients are diagnosed with chronic venous insufficiency, and nearly $500 million is used in the care of these patients. If left untreated, CVI is usually progressive and leads to postphlebitic syndrome and venous ulcers.
The approach to CVI management involves several different strategies, including conservative therapies (eg, compression, elevation, and exercise), pharmacologic treatments, and more invasive procedures (eg, sclerotherapy, endovenous laser or radiofrequency ablation, or surgical ligation). Delayed treatment can result in a more rapid disease progression, leading to complications including venous ulcers, infection, and deep vein thrombosis. This activity for healthcare professionals aims to enhance learners' competence in selecting appropriate diagnostic tests, managing chronic venous insufficiency, and fostering effective interprofessional teamwork to improve outcomes.
Objectives:
Assess the various etiologies and risk factors for chronic venous insufficiency.
Identify the clinical features of chronic venous insufficiency.
Select the appropriate management approach for chronic venous insufficiency.
Implement interprofessional team strategies to improve care and optimize outcomes for patients affected by chronic venous insufficiency.
Introduction
Chronic venous insufficiency (CVI) is a condition in which the flow of superficial or deep venous blood is impaired, causing venous hypertension. CVI encompasses several pathological changes (eg, lower extremity edema, skin trophic changes, and discomfort) that result secondary to venous hypertension.[1][2] Chronic venous insufficiency is a prevalent disease process around the world. Disability associated with chronic venous insufficiency contributes to a diminished quality of life and loss of work productivity. In most cases, the underlying cause is incompetent venous valves. Each year, approximately 150,000 new patients are diagnosed with chronic venous insufficiency, and nearly $500 million is used in the care of these patients. If left untreated, CVI is usually progressive and leads to postphlebitic syndrome and venous ulcers. Other common symptoms include pain, leg swelling, pruritus, skin discoloration, limb heaviness, and edema that improves with elevation.[3]
The diagnosis of CVI is based on clinical features and confirmatory diagnostic studies. An international consensus conference developed the Clinical, Etiology, Anatomic, and Pathophysiology (CEAP) classification to improve consistency in reporting, diagnosis, and management of CVI.[4][5] Venous duplex ultrasound is the primary modality to confirm a CVI diagnosis and is considered the gold standard. In addition to complementing the CEAP, the revised Venous Clinical Severity Score was developed to help assess CVI severity and determine the efficacy of CVD treatments.[6][7][8][9][5] The approach to CVI management involves several different strategies, including conservative therapies (eg, compression, elevation, and exercise), pharmacologic treatments, and more invasive procedures (eg, sclerotherapy, endovenous laser or radiofrequency ablation, or surgical ligation).[5] Delayed treatment can result in a more rapid disease progression, leading to complications including venous ulcers, infection, and deep vein thrombosis.[5][3][1]
Etiology
Though the exact etiology of CVI is unclear, the primary underlying mechanism is believed to be valvular reflux. Other CVI etiologies include venous outflow obstruction, arteriovenous malformation, and calf muscle pump failure. There may also be a hereditary component since genetic disorders like Klippel-Trenaunay and Parkes-Weber are known to cause CVI.[10] These etiologic factors can lead to chronic endothelial inflammation and other pathophysiologic changes.[2][3]
Chronic Venous Insufficiency Risk Factors
The following risk factors are associated with CVI development:
- Advanced age (ie, aged 55 or older)
- Family history of varicose veins or venous pathology
- Obesity
- Oral contraceptive use
- Tobacco use
- Pregnancy
- History of deep vein thrombosis or thrombophlebitis
- History of the leg injury
- Prolonged standing or sitting
- Sedentary lifestyle
- Female sex [5][3]
Epidemiology
An estimated 10% to 35% of adults have CVI in the US, with 4% of adults aged 65 years or older developing venous ulcers.[3] Results across studies suggest that in the general population, between 1% and 17% of men and 1% and 40% of women may experience chronic venous insufficiency. Furthermore, the prevalence of varicose veins has been reported to range from under 1% to 70%. The variation in these estimations is likely due to the different diagnostic criteria used and the population surveyed.[1] Globally, the prevalence of CVI is higher in industrialized nations (eg, Western Europe and the US) due to more inactive lifestyles.[5]
The prevalence of venous ulcers, a common complication of CVI, in the US ranges from approximately 1% to 3%.[3] Similarly, lower leg ulceration occurs in approximately 1% to 2% of the global adult population and is reported to increase to 3% in patients older than 65.[5] Formation of an ulcer carries a poor prognosis, with 40% of patients developing recurrence despite standard treatment. Management of chronic venous insufficiency accounts for approximately 2% of the US's total healthcare.[11][12]
Pathophysiology
The peripheral venous system serves as a blood reserve and a channel for returning blood to the heart. The patency of blood veins, valves, and muscle pumps is necessary to function the venous system properly. To return to the central circulation, blood must travel against gravity and other pressures in the upright position. The veins of the lower extremities are classified as superficial (above the facial muscle layer), deep (below the fascial layer), and perforator.[13] The superficial venous system consists of the great saphenous vein (GSV), small saphenous vein, and several accessory veins. Deep veins are made up of axial veins. Perforating veins traverse the fascial layer, connecting superficially to deep veins. A number of one-way bicuspid valves throughout the deep and superficial veins allow blood to travel toward the heart while preventing it from returning to the feet.[14] Chronic venous insufficiency pathophysiology is either due to reflux (ie, backward flow) or obstruction of venous blood flow. Chronic venous insufficiency can develop from the protracted valvular incompetence of superficial, deep, or perforating veins that connect them. In all cases, the result is venous hypertension of the lower extremities. The resting venous pressure is a summation of the outflow obstruction, capillary inflow, valve function, and muscle pump function.
Superficial incompetence is usually due to weakened or abnormally shaped valves or widened venous diameters, which prevents normal valve congruence. In most cases, the leaky valve is located near the termination of the greater saphenous vein into the common femoral vein. Deep vein dysfunction usually owes to the previous deep vein thrombosis (DVT), which results in inflammation, valve scarring and adhesion, and luminal narrowing. Perforating vein valvular failure allows higher pressure to enter the superficial venous system. The subsequent dilation prevents the proper closure of the valve cusps in the superficial veins. Most patients will also have the disease in the superficial veins.
Primary chronic venous insufficiency is the symptomatic presentation without a precipitating event due to congenital disabilities or changes in venous wall biochemistry. Recent studies suggest that approximately 70% of patients have primary chronic venous insufficiency, and 30% have secondary disease. Studies into primary chronic venous insufficiency have identified reduced elastin content, increased extracellular matrix remodeling, and inflammatory infiltrate. The culmination alters the vein's integrity, promoting dilation and valvular incompetence. Secondary chronic venous insufficiency occurs in response to a DVT, which triggers an inflammatory response, subsequently injuring the vein wall. Irrespective of the specific etiology, chronic venous insufficiency promotes venous hypertension. The most common nonmodifiable risk factors are female gender and nonthrombotic iliac vein obstruction (eg, May-Thurner syndrome). Several studies have also suggested a genetic component contributing to vein wall laxity. Modifiable risk factors include smoking, obesity, pregnancy, prolonged standing, DVT, and venous injury.[15][16] Regardless of the cause, the persistently elevated venous hydrostatic pressure may result in lower extremity pain, edema, and venous microangiopathy. Some patients develop permanent skin hyperpigmentation from hemosiderin deposition as red blood cells extravasate into the surrounding tissue. Many of these patients will also have lipodermatosclerosis, skin thickening from subcutaneous fat fibrosis. As the disease progresses, the perturbed microcirculation and dermal weakening can form ulcers.[17]
History and Physical
Clinical Features
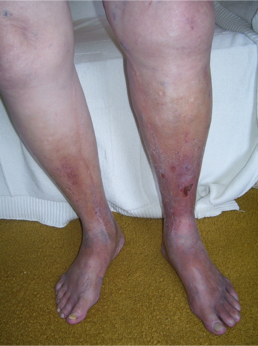
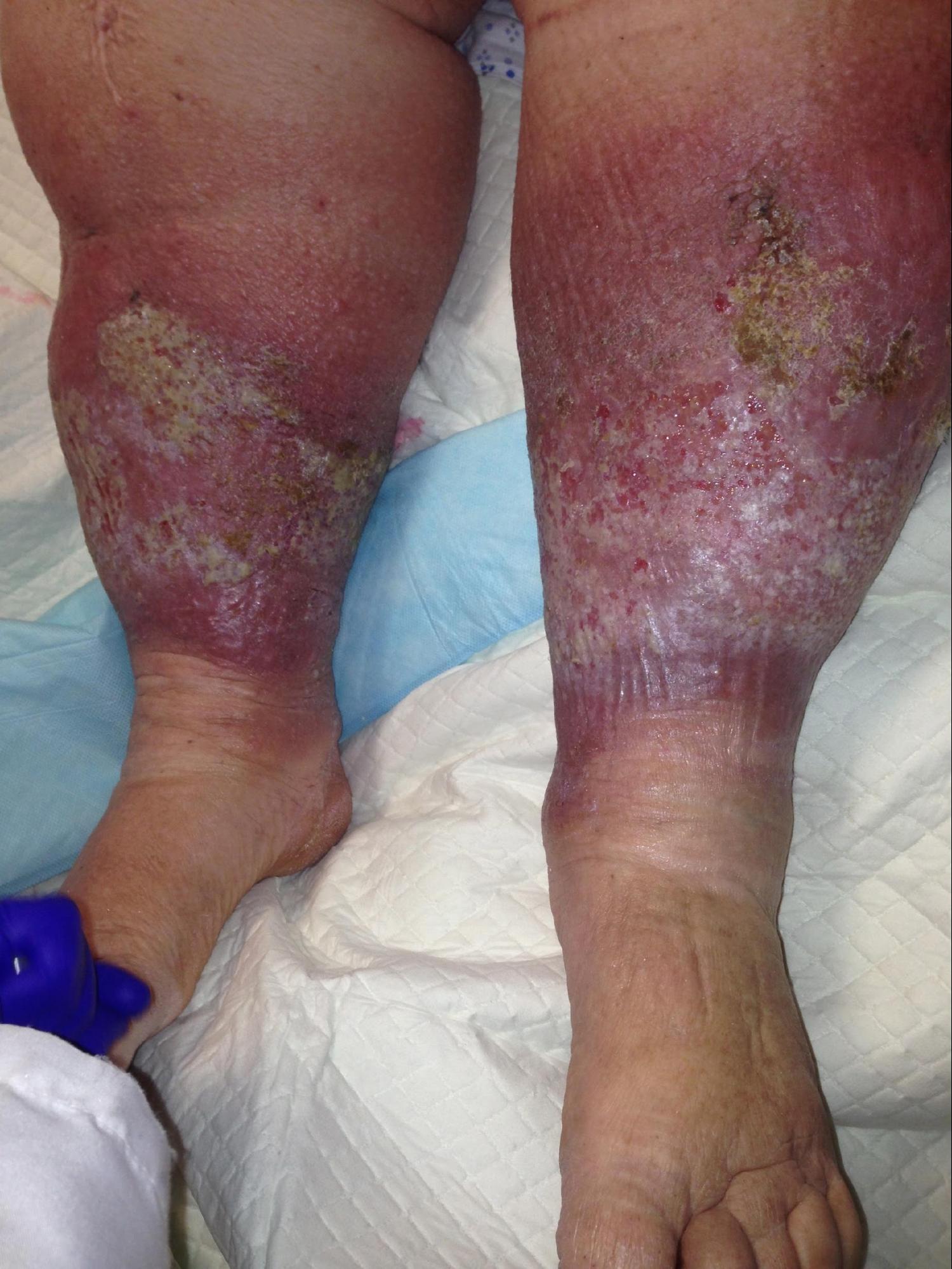
Patients with chronic venous insufficiency (CVI) commonly present initially with a combination of dependent pitting edema, leg discomfort, fatigue, and itching. Although there can be variations in presentation among patients, certain features are more prevalent, including telangiectasias, reticular veins, varicose veins, pain, cramping, itching, prickling, and throbbing sensation. Patients may describe symptoms that improve with rest and leg elevation and with no association with exercise. This latter feature can be used to distinguish venous from arterial claudication. As their disease progresses, varicose veins, tenderness, refractory edema, and skin changes can be noted. (see Image. Chronic Venous Insufficiency) Patients with advanced disease will present with a severe blanched skin lesion, dermal atrophy, hyperpigmentation, dilated venous capillaries, and ulcer formation, most commonly overlying the medial malleolus. A thorough history should note any hypercoagulable condition, oral contraceptive use, previous DVT or intervention, level of physical activity, and occupation. The patient's presentation should carefully be distinguished from other pathologies with similar symptoms: diabetic ulcers, ischemic ulcers, and dermatologic conditions, including cancer.[4][5][3][5]
The physical exam should involve a detailed assessment of any ulcers, distal pulses, and neuropathy in the upright and supine positions. The Trendelenburg test may help differentiate between the CVI caused by the superficial vein valves and the deep system. The patient's leg is elevated to perform the test, and all the venous blood is emptied. The surgeon then compresses the groin firmly to occlude the greater saphenous vein junction and asks the patient to stand up. If the leg does not fill up with venous blood, this indicates that incompetent valves in the superficial veins cause CVI. If the leg fills with venous blood, the valves connecting the superficial veins to the deep ones are incompetent. The treatment is limited to compression stockings if the deep system valves are involved.[4][5][3][5]
Clinical, Etiology, Anatomic, and Pathophysiology Classification
An international consensus conference developed the following Clinical, Etiology, Anatomic, and Pathophysiology (CEAP) classification to improve consistency in reporting, diagnosis, and management of CVI.[4][5] Additionally, the revised Venous Clinical Severity Score (VCSS) is another clinical tool developed to help assess CVI severity and determine the efficacy of CVD treatments. The VCSS grades 10 CVI clinical features from absent to severe, including pain, varicose veins, edema, skin pigmentation, and ulcer characteristics (eg, number and size). This scoring system can be used to complement the CEAP during clinical assessment.[6][7][8][9][5]
- Clinical classification
- C0: No visible or palpable signs of venous disease
- C1: Telangiectasias or reticular veins
- C2: Varicose veins
- C3: Edema
- C4a: Pigmentation and eczema
- C4b: Lipodermatosclerosis and atrophy (see Image. Chronic Venous Insufficiency and Lipodermatosclerosis).
- C5: Healed venous ulcer
- C6: Active venous ulcer
- Etiologic classification
- Ec: Congenital
- Ep: Primary
- Es: Secondary
- En: No venous etiology identified
- Anatomic classification
- As: Superficial veins
- Ap: Perforator veins
- Ad: Deep veins
- An: No venous location identified
- Pathophysiologic classification
- Pr: Reflux
- P0: Obstruction
- Pr/o: Reflux and obstruction
- Pn: No venous pathophysiology identifiable
Evaluation
The diagnosis of CVI is based on clinical features and confirmatory diagnostic studies. Venous duplex ultrasound is the primary modality to confirm a CVI diagnosis; however, several other ancillary studies may also be considered.
Venous Duplex Ultrasound
When evaluating CVI, color duplex ultrasound is considered the gold standard. Guidelines developed by a consensus of the American Venous Forum, the Society for Vascular Surgery, the American Vein and Lymphatic Society, and the Society of Interventional Radiology recommended that patients be in the upright position in individuals with the capability to stand safely when a duplex ultrasound is performed to evaluate venous reflux. Evaluation in the supine or steep reverse Trendelenburg positions was not preferred. A Doppler waveform reflux duration in the superficial veins of >0.5 sec and >1.0 seconds in the deep veins (eg, femoral and popliteal) was used as the diagnostic threshold for venous reflux of an incompetent valve. To help induce venous reflux, distal compression at the calf may be performed. Doppler ultrasound with color flow may help visualize venous flow patterns.[5]
Air Plethysmography
Air plethysmography (APG) can assess all potential pathophysiologic processes of CVI (eg, reflux, obstruction, and muscle pump failure) by measuring the air displaced in a cuff wrapped around the calf while moving the legs through various positions and exercises. The air displaced correlates to the venous volume and refilling time. The APG is utilized primarily when the venous duplex ultrasound evaluation is equivocal or inadequate.[18] Abnormal venous filling indices correspond with the severity of CVI, provide information on several features of global venous function, and can be utilized for intervention selection and response evaluation.[19][20]
Ancillary Diagnostic Studies
Computed tomography and magnetic resonance venography require intravenous contrast material and are particularly useful for evaluating proximal veins and their surrounding structures to assess for intrinsic and extrinsic compression. Before intervention is advised, these procedures may be utilized to characterize complex venous anatomy, such as an iliofemoral venous blockage.[14] Photoplethysmography measures venous filling times by the amount of reflected infrared light of hemoglobin. This modality is typically used for subcutaneous veins. Other noninvasive modalities include strain gauge plethysmography and foot volumetry.
Invasive diagnostic tests that may be considered include contrast venography, which is most beneficial in identifying reflux in the common femoral vein and saphenofemoral junction, and in cases of venous reconstruction, intravascular ultrasound, which is a catheter-based ultrasound probe employed to visualize periluminal vascular anatomy to detect venous obstruction or stenosis. Ambulatory venous pressure measurement is the gold standard for determining the hemodynamics of CVI; it requires inserting a needle into the dorsal foot vein and connecting it to a pressure transducer.[21] Despite its value in determining CVI's severity and clinical outcomes, it is rarely used because of its invasive nature and alternative diagnostic modalities.
Treatment / Management
Patients with chronic venous insufficiency should be treated based on the severity and nature of the disease. The primary goals of management are to reduce discomfort and edema, stabilize skin appearance, reduce venous reflux and varicose veins, and heal ulcers. The recommended management approach is to utilize conservative treatments (eg, leg elevation, resistance exercises, weight management, and compression therapy) alone or in conjunction with other therapies, including medications and interventional procedures.
Compression Therapy and Other Conservative Strategies
Compression treatment is the main component of the conservative approach, which aims to provide graduated external compression to the lower extremities and counteract the hydrostatic pressures of venous hypertension. Available compression garments comprise graded elastic compressive stockings, gauze boots, layered bandaging, and adjustable compression garments.[14] Compression strengths between 30 and 50 mm Hg are believed to improve venous reflux, pain, edema, pigmentation, ulcer healing, and prevention, with 70% and 80% compliance rates.[22] Compression therapy is long-term and only benefits patients who remain compliant. Ulcers are treated best with compression bandaging systems. Chronic venous ulcerations entail a risk of infection and cancerous transformation (eg, Marjolin ulcer). Compression therapy should be used with caution in patients with coexisting peripheral arterial disease. Severe arterial insufficiency and uncompensated congestive heart failure are contraindications to compression therapy. Patients whose ulcers fail to respond to compression may ultimately need surgical intervention.
Other conservative treatments include leg elevation, weight management, and exercise. Leg elevation above the heart for at least 30 min 3 times a day helps to reduce venous pressure and edema and prevent recurrent ulcers. Obesity is a well-established risk factor for developing CVI and associated complications; thus, maintaining an optimal body weight may improve CVI symptoms.[23] Resistance exercises help improve the calf muscle pump, which normally aids venous blood return from the lower extremities in healthy individuals and is impaired in those with CVI. Activities that involve ankle dorsiflexion, ankle plantarflexion, and forceful toe flexion (eg, walking and cycling) are the most effective movements for increasing systolic blood velocity.[3][1]
Proper skin and wound care are essential in conjunction with other conservative therapies. Advanced CVI may affect skin integrity; therefore, maintaining skin health and avoiding infection is crucial. Topical moisturizers, commonly containing lanolin, minimize skin fissuring and disintegration. Topical steroids may be used to treat stasis dermatitis. Bacterial overgrowth may occur with venous ulcers; hence, diligent wound care is needed to limit infection. Hydrocolloids and foam dressings can reduce wound fluid drainage and skin maceration.[24] Biologic skin replacements derived from tissue engineering have been used to treat ulcers with some effectiveness, although silver-impregnated dressing has been controversial.
Interventional Procedures
Superficial vein reflux can be managed with foam sclerotherapy, endovenous thermal ablation, or stripping. Deep vein reflux may be treated with valve reconstruction or valve transplant. Perforator reflux can be managed with sclerotherapy, endovenous thermal ablation, or subfascial endoscopic perforator surgery (SEPS). However, compression therapy regimens that are adhered to are highly effective in treating all forms of venous pathophysiology.[9][25][26]
- Sclerotherapy: Sclerotherapy is beneficial in treating telangiectasias, reticular veins, varicose veins 1 to 4 mm in diameter, and venous reflux. This treatment modality can be used alone or with other treatments. Sclerosing agents include the hypertonic solution of sodium chloride (23.4%), polidocanol, sodium iodide, chromated glycerin, sodium tetradecyl sulfate, and sodium morrhuate. The sclerosing agent must often be diluted for veins with a lower diameter to prevent tissue irritation and necrosis. Polidocanol is superior to normal saline, eliminating incompetent varicose veins and enhancing venous hemodynamics. However, sclerosants are used to manage spider veins, not varicose veins, as a large amount of solution would be required to collapse a varicosity, leading to extreme pain, thrombosis, and permanent skin discoloration.[27] Darkening of the surrounding skin because of hemosiderin degradation is a common side effect of sclerotherapy, which may be mitigated by microthrombectomy, which involves the expulsion of the thrombus.
- Endovenous ablative therapy: Ablation of incompetent veins utilizes thermal energy through radiofrequency or laser. This procedure is often used for venous reflux as an alternative to stripping. The heat produced induces local thermal damage to the vein wall, leading to thrombosis and fibrosis. Venous radiofrequency ablation results in complete obliteration in 85% of patients after 2 years. Laser therapy with an 810 nm or 940 nm diode has shown outstanding outcomes, with saphenous vein obliteration in 93% of patients at 2 years.[28] Both radiofrequency and laser treatments are administered under tumescent anesthesia to avoid skin burns, minimize discomfort, and facilitate a speedier return to regular activities. Deep venous thrombosis and pulmonary embolism remain, although seldom, the possible complications of ablation.
- Endovenous deep system therapy: For iliac vein stenosis and occlusion, endovascular stenting has superseded surgical techniques such as cross-femoral venous bypass or prosthetic iliac vein reconstructions. Close monitoring is recommended to maintain stent patency since in-stent restenosis or occlusion can occur in individuals with thrombotic illness; however, restenosis is uncommon.[14] The efficacy of iliac stenting seems to be lasting, with 85% to 90% of patients being free of recurrent ulcers after 5 years.[29]
- Surgical management: In individuals who do not respond to pharmacological or endovenous therapy, surgical surgery for CVI may be considered in addition to compression stockings. Surgical treatment may be beneficial for individuals with persistent discomfort and disability, recurrent varicose veins, inability to cooperate with compression treatment, and chronic nonhealing venous ulcers. The surgical method depends on the underlying pathophysiologic processes and the afflicted vein area. Ligation and stripping have been shown to enhance venous hemodynamics, alleviate pain, and promote ulcer healing. Venous valve reconstruction procedures include valvuloplasty, transposition, transplant, cryopreserved vein valve allografts, and neo-valve construction. Valvuloplasty is done in some centers, but the surgery is technically demanding and does not always work. No matter which surgery is selected, patients should combine it with compression stockings for maximal effectiveness. Complications related to surgery include:[3][1][3]
- Infection
- Injury to the arterial system
- Nerve injury (eg, saphenous and or sural nerves)
- Poor cosmesis
- Deep vein thrombosis
- Scarring
Differential Diagnosis
Other diagnoses that should also be considered include:
- Lymphedema
- Cellulitis
- Stasis dermatitis
- Varicose veins
- Acute deep vein thrombosis
- Heart failure
- Cirrhosis
- Renal failure
- Endocrine disorders (eg, hypothyroidism)
- Medication side effects (eg, calcium channel blockers, NSAIDs, and oral hypoglycemic agents)
- Lipedema
- Ruptured popliteal cyst
- Soft tissue hematoma or mass
- Exertional compartment syndrome
- Gastrocnemius tear
Prognosis
CVI is not a benign disorder and carries enormous morbidity. Without correction, the condition is progressive. Venous ulcers are common and very difficult to treat. Chronic venous ulcers are painful and debilitating. Even with treatment, recurrences are common if venous hypertension persists. Nearly 60% develop phlebitis, which often progresses to deep vein thrombosis in more than 50% of patients. The venous insufficiency can also lead to severe hemorrhage. Surgery for CVI remains unsatisfactory despite the availability of numerous procedures. The financial cost of care to the patient can also be enormous.[3]
Complications
Chronic venous insufficiency is associated with the following complications:
- Chronic venous ulceration
- Deep vein thrombosis
- Recurrent cellulitis
- Lipodermatosclerosis
- Secondary lymphedema
- Stasis dermatitis
- Chronic pain
- Superficial thrombophlebitis
- Secondary hemorrhage
- Atrophie blanche
- Ankle joint stiffness from chronic scarring
Deterrence and Patient Education
The patient should be instructed on effectively and adequately using compression stockings, emphasizing compliance and using the optimal tension gradient. Compression stockings alone may ease discomfort, edema, and venous distention, aid venous ulcer healing, and prevent recurrences. In addition, the patient should be instructed to preserve skin integrity by regularly checking the skin for breakdowns or infections and diligently applying moisturizer to prevent fissuring. Also, patients should be advised to elevate their legs to minimize swelling and avoid extended periods of standing or sitting. Patients should also be encouraged to maintain ideal body weight and identify any obstacles that hamper losing weight, including mental health issues (eg, depression, anxiety, and eating disorders), medications causing weight gain, or mobility issues. An appropriate specialist referral or patient counseling should address these concerns. In addition, patients should be made aware that chronic venous disease is a long-term health concern. Hence, regular follow-up with clinicians and compliance with medical treatment plans are essential for preventing its consequences.
Enhancing Healthcare Team Outcomes
The optimum chronic venous disease treatment requires an interprofessional team approach, including primary care physicians, vascular and general surgery experts, wound care nurses, physical and occupational therapists, dieticians, a weight management team, bariatric surgery, and pharmacists. An early diagnosis, treatment plan, and referral to a specialist are crucial to effectively managing chronic venous disease. Primary care clinicians should conduct diagnostic testing to rule out other causes of lower limb edema and avoid prescribing medications that may exacerbate the illness, such as calcium channel blockers and nonsteroidal anti-inflammatory medicines. The pharmacist may be able to review the patient's drugs and aid in the development of an alternate treatment plan to prevent the condition from worsening. Wound care nurses and occupational therapists may aid in treating venous ulcers. Chronic venous ulcers that do not heal or disease recurrence should demand referral to vascular and general surgery experts. To avoid disease progression and recurrence, patients should be closely followed. Patients who fail conservative or pharmacological approaches to losing weight should be referred to a bariatric surgeon, as losing weight/maintaining optimal weight is crucial in managing chronic venous disease.