Introduction
Preparing the wound bed to promote reepithelialization of chronic wounds has been applied to wound management for over a decade. The four general steps to follow for better preparation are compassed in the acronym DIME.[1][2][3][4][5]
- D: Debridement of nonviable tissue within the Wound.
- I: Management of Inflammation and Infection
- M: Moisture control
- E: Environmental and Epithelialization assessment
The DIME approach to chronic wound management is a global concept approach from which a more detailed pathway can be initiated to bring about wound resolution.
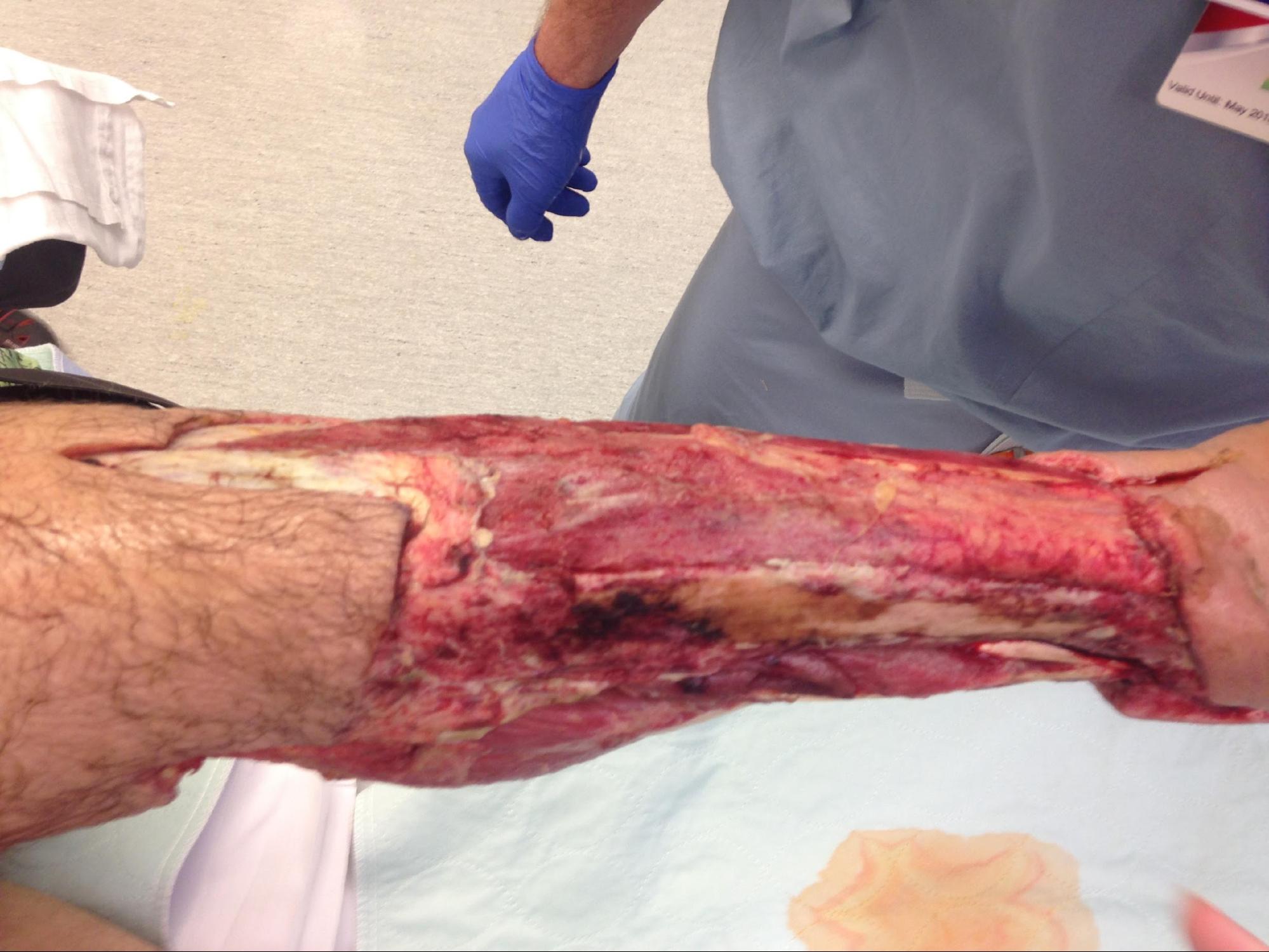
The primary goal of debridement is to remove all the devitalized tissue from the wound bed to promote wound healing. Debridement is also used for the removal of biofilm, bioburden along with senescent cells, and it is suggested to be performed at each encounter.[6][7][8]
Anatomy and Physiology
The skin's two main layers are the epidermis and the dermis. The epidermis comprises closely packed epithelial cells, and the dermis is composed of dense, irregular connective tissue where the blood vessels, hair follicles, sweat glands, and other structures are housed. The hypodermis lies beneath the dermis. Its composition is mostly loose connective and fatty tissues. Muscles, tendons, ligaments, bones, and cartilage are all under hypodermis.
The epidermis is composed of keratinized, stratified, squamous epithelium. The dermis contains blood, lymph vessels, nerves, and other structures, such as hair follicles and sweat glands.[9]
Indications
In general, the indication for debridement is the removal of devitalized tissue such as necrotic tissue, slough, bioburden, biofilm, and apoptotic cells.[10]
Debridement is a major component of wound management to prepare the wound bed for re-epithelialization. Devitalized tissue, in general, and necrotic tissue, in particular, serve as the source of nutrients for bacteria. Devitalized tissue also acts as a physical barrier for re-epithelialization, preventing applied topical compounds to make direct contact with the wound bed to provide their beneficial properties. Necrotic tissue also prevents angiogenesis, granulation tissue formation, epidermal resurfacing, and normal extracellular matrix (ECM) formation. Finally, the presence of necrotic tissue may prevent the clinician from accurately assessing the wound's extent and severity, even masking possible underlying infections.[11][12][13]
Schiffman et al. include the following as common indications for sharp surgical debridement.
- Removal of the source of sepsis, mainly necrotic tissue
- Removal of local infection to decrease bacterial burden, reduce the probability of resistance from antibiotic treatment, and obtain accurate cultures
- Collection of deep cultures taken after debridement from the tissue left behind to evaluate persistent infection and requirements for systemic antibiotic treatment
- Stimulation of the wound bed to support healing and to prepare for a skin graft or flap[14]
Contraindications
Contraindication of wound debridement, in general, may be applied to dry and intact eschars with no clinical evidence of underlying infection, such as with an unstageable pressure ulcer with an intact eschar at the sacrum or buttock, or heel.
Other contraindications pertain to each particular method of debridement.[15]
Technique or Treatment
Several types of debridements can achieve the removal of devitalized tissue. These include surgical debridement, biological debridement, enzymatic debridements, and autolytic debridement.
Autolytic Debridement
This is the most conservative type of debridement. This debridement is a natural process by which endogenous phagocytic cells and proteolytic enzymes break down necrotic tissue. It is a highly selective process whereby only necrotic tissue will be affected in the debridement.
It is indicated for noninfected wounds. It may also be used as adjunctive therapy in infected wounds. It can be used with other debridement techniques, such as mechanical debridement in the case of infected wounds.
It requires a moist environment and a functional immune system. The use of moisture-retentive dressings can enhance it. This debridement induces softening of the necrotic tissue and eventual separation from the wound bed.
The effectiveness of this type of debridement is mandated by the amount of devitalized tissue to be removed and the actual wound size.
Autolytic debridement will take a few days. If a significant decrease in necrotic tissue is not seen within 1 or 2 days, a different debridement method should be considered.[16]
Biological Debridement
Biological debridement, also known as larval therapy, uses sterile larvae of the Lucilia sericata species of the green bottle fly. It is an effective mode of debridement, particularly appropriate in large wounds where a painless removal of necrotic tissue is needed. The mechanism of action of mega therapy/debridement consists mainly of the release of proteolytic enzymes containing secretions and excretions that dissolve necrotic tissue from the wound bed. Other modes of action contributing to the overall result of larval therapy are:[17]
- Bacteriocidal, as the larvae ingest and digest bacteria
- Inhibiting bacterial growth by producing in releasing ammonia into the wound bed, which increases the wound pH
- Breakdown of existing biofilm at the wound bed and inhibition of new biofilm growth
- Direct ingestion of necrotic tissue[18]
Chitosan, the major derivative of chitin, has been widely investigated for biomedical applications for several reasons, including, but not limited to, 1. highly porous structure, 2. its strong biocompatibility profile, and 3. its intrinsic antibacterial nature. [19][20] Moreover, composites of chitosan induce tissue regeneration. Former research demonstrated that chitosan nanofibrous scaffolds could be potentially utilized to masquerade a biocompatible extracellular matrix. The resultant ECM would support the infrastructure of different cell types, including smooth muscle cells, neural cells, and fibroblasts.[21][19]
It should be noted that chitosan alone cannot be applied for the purpose of ECM replacement due to its limited mechanical properties. Therefore, to provide nanofibrous scaffolds, chitosan should be combined with synthetic polymers, specifically electro-spun polymers, including polycaprolactone (PCL).[22] PCL is a synthetic polymer with excellent electrospinnability and a favorable mechanical profile.
Maggots can be applied to the wound bed. They can be enclosed in a biological bag or are in free range.
Studies have shown that free-range maggots can debride a wound at least twice as fast as bag-pain maggots. Comparison studies of either free-range maggots treatment versus bio bag contained maggots versus hydrogel autolytic debridement shows days to complete debridement to be 14 versus 28 versus 72 days, respectively.
Contraindications to biological debridement are an abdominal wound contiguous with the intraperitoneal cavity, pyoderma gangrenosum in patients with immunosuppression therapy, and wounds in proximity to areas afflicted by septic arthritis.
Enzymatic Debridement
This is a selective method for debridement of necrotic tissue using an exogenous proteolytic enzyme, collagenase, to debride Clostridium bacteria. Collagenase digests the collagen in the necrotic tissue allowing it to detach.
Enzymatic debridement is a slow method of debridement from hair to mechanical and sharp debridement.
Collagenase and moisture retentive dressings can work in synergy, enhancing the debridement.
Enzymatic debridement is not recommended for an advanced process or in patients with known sensitivity to the product's ingredients.
A relative contraindication of enzymatic debridement is its use in heavily infected wounds. Furthermore, collagenase should not be used in conjunction with silver-based products or with Dakin solution.[23]
Surgical Debridement with Sharp Instruments
This is a type of debridement where devitalized tissue (slough, necrotic, or eschar) in the presence of underlying infection is removed using sharp instruments such as a scalpel, Metzenbaum, and curettes, among others. This can be done bedside, in the office or wound care center, or the operating room, depending on the adequacy of anesthesia and the ability to control perioperative complications like bleeding. The healthcare professional should be skilled, trained, qualified, and licensed to provide surgical treatment.
Sharp-instrument debridement can be combined with all the other debridement methods during the perioperative period.
Disadvantages of surgical debridement include adverse events from the debridement itself, for example, bleeding and possible general complications from the anesthesia.
Contraindications for surgical debridement in the operating room would have to take into account the particular surgical risk stratification of the patient. Sharp surgical debridement is contraindicated in patients with an intact eschar and no clinical evidence of an underlying infection. In these cases, the intact eschar is a biological covering for the underlying skin defect. This is usually seen in unstageable pressure injuries at the sacrum, buttocks, or heels with intact and/or dry eschars.[24]
Mechanical Debridement
Mechanical debridement is a nonselective type of debridement, meaning that it will remove both devitalized tissue and debris and viable tissue. It is usually carried out using mechanical force: wet-to-dry, pulsatile lavage, or wound irrigation.
It is indicated for acute and chronic wounds with moderate to large amounts of necrotic tissue, regardless of the presence of an active infection.
The contraindications include, depending on the modality of mechanical debridement used, the presence of granulation tissue in a higher amount than the devitalized tissue, inability to control pain, patients with poor perfusion, and an intact eschar with no gross clinical evidence of an underlying infection.
Complications
Depending on the type of debridement chosen, complications range from local irritation to major bleeding.
Surgical debridement and mechanical debridement will have a higher risk for bleeding, along with peri-procedural pain.
Surgical debridement done in the operating room must account for morbidity and mortality due to the anesthesia used. A study by J. Shiffman et al. has shown operative mortality to be 2% with long-term mortality and as high as 68% following debridement.
Clinical Significance
The clinical significance of wound debridement and ulcers with necrotic tissue, regardless of the infection status, cannot be overstated and should not be underestimated. Debridement for most wounds is considered a standard in wound management. It provides the benefits of removal of necrotic tissue and bacteria and senescent cells, as well as the stimulating activity of growth factors. Sharp surgical debridement has been shown to reset the proper timing of the phases of wound re-epithelialization by providing the initial trauma seen in the hemostasis phase of wound healing. Angiogenesis has also been shown to be stimulated by sharp surgical debridement.
Of course, not all forms of debridement will have the same impact on the wound or the ulcer as the mode of action differs; however, either sharp surgical debridement or nonsurgical debridement is fundamental to wound re-epithelialization.
A critical concept of debridement is the dry eschar, such as in unstageable pressure ulcers with no overt signs of infection, where debridement is not always indicated because the dry eschar will act as a biological covering.
The mode of debridement should be tailored to the particular wound presentation, considering factors such as comorbidities, other lower-risk options, and the patient's comfort and desires.[25][26]
Enhancing Healthcare Team Outcomes
The management of chronic wounds is done by an interprofessional team that consists of a general surgeon, vascular surgeon, wound care nurse, infectious disease expert, physical therapist, dietitian, and internist. There are many types of wounds caused by several factors. When wounds do not heal, it is important to consult with a wound care specialist. There are dozens of methods of treating wounds, but the key is to ensure that the wound remains free of contamination and bacteria. The outcome of chronic wounds depends on the cause. Many chronic wounds can take weeks, months, or years to finally close. At the same time, the patient's nutrition and functional ability must be continually assessed.[27][28]