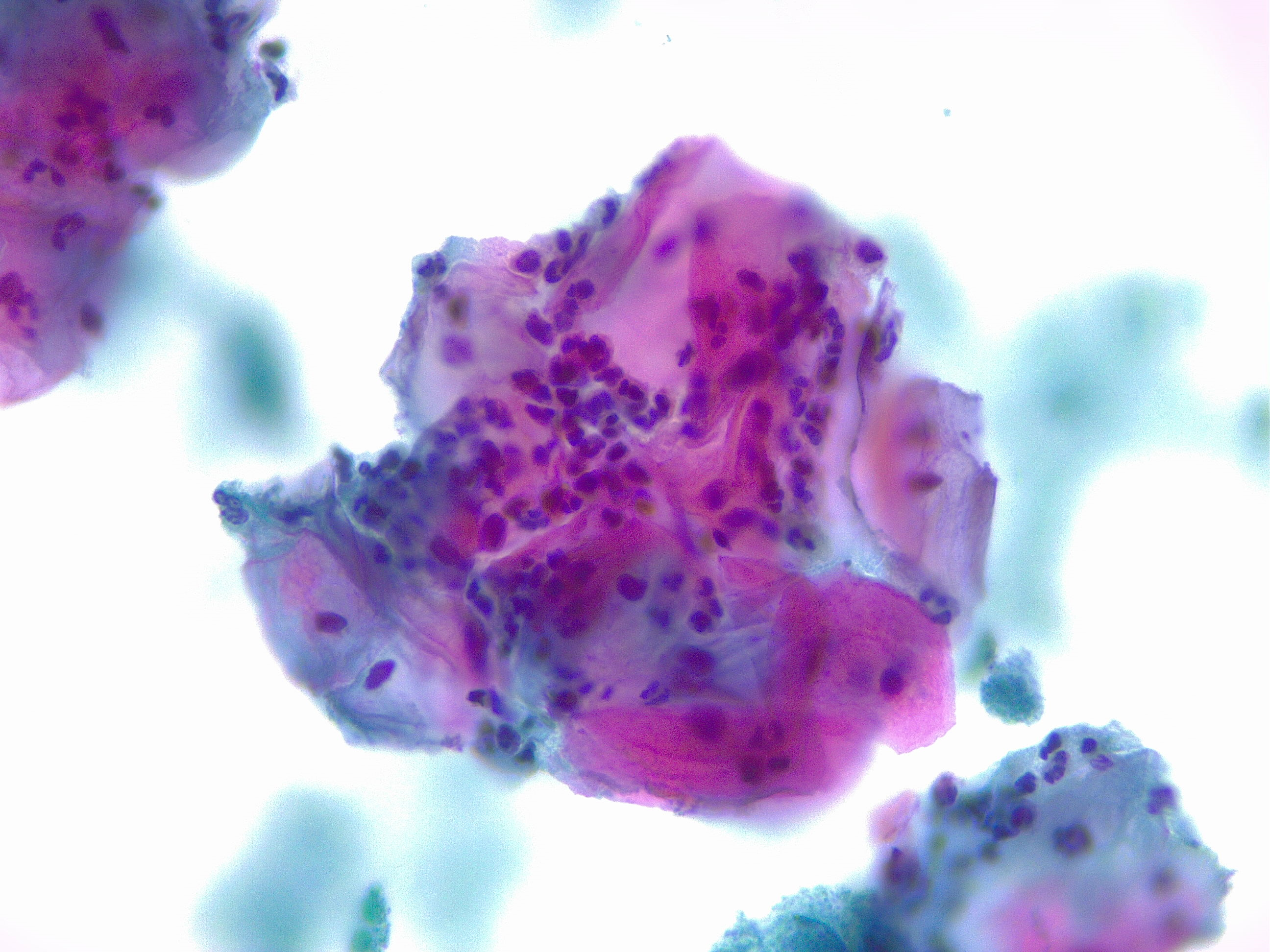
[1]
Sanford NN, Sher DJ, Butler S, Xu X, Ahn C, D'Amico AV, Rebbeck T, Aizer AA, Mahal BA. Cancer Screening Patterns Among Current, Former, and Never Smokers in the United States, 2010-2015. JAMA network open. 2019 May 3:2(5):e193759. doi: 10.1001/jamanetworkopen.2019.3759. Epub 2019 May 3
[PubMed PMID: 31099863]
[2]
Nogueira-Rodrigues A. HPV Vaccination in Latin America: Global Challenges and Feasible Solutions. American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Annual Meeting. 2019 Jan:39():e45-e52. doi: 10.1200/EDBK_249695. Epub 2019 May 17
[PubMed PMID: 31099692]
[3]
Ge Y, Mody RR, Olsen RJ, Zhou H, Luna E, Armylagos D, Puntachart N, Hendrickson H, Schwartz MR, Mody DR. HPV status in women with high-grade dysplasia on cervical biopsy and preceding negative HPV tests. Journal of the American Society of Cytopathology. 2019 May-Jun:8(3):149-156. doi: 10.1016/j.jasc.2019.01.001. Epub 2019 Jan 14
[PubMed PMID: 31097291]
[4]
Niu S,Molberg K,Thibodeaux J,Rivera-Colon G,Hinson S,Zheng W,Lucas E, Challenges in the Pap diagnosis of endocervical adenocarcinoma in situ. Journal of the American Society of Cytopathology. 2019 May - Jun;
[PubMed PMID: 31097290]
[5]
Rukhadze L, Lunet N, Peleteiro B. Cervical cytology use in Portugal: Results from the National Health Survey 2014. The journal of obstetrics and gynaecology research. 2019 Jul:45(7):1286-1295. doi: 10.1111/jog.13974. Epub 2019 Apr 29
[PubMed PMID: 31034140]
Level 3 (low-level) evidence
[6]
Swailes AL, Hossler CE, Kesterson JP. Pathway to the Papanicolaou smear: The development of cervical cytology in twentieth-century America and implications in the present day. Gynecologic oncology. 2019 Jul:154(1):3-7. doi: 10.1016/j.ygyno.2019.04.004. Epub 2019 Apr 15
[PubMed PMID: 30995961]
[7]
Corkum MT, Shaddick H, Jewlal E, Patil N, Leung E, Sugimoto A, McGee J, Prefontaine M, D'Souza D. When Pap Testing Fails to Prevent Cervix Cancer: A Qualitative Study of the Experience of Screened Women Under 50 with Advanced Cervix Cancer in Canada. Cureus. 2019 Jan 24:11(1):e3950. doi: 10.7759/cureus.3950. Epub 2019 Jan 24
[PubMed PMID: 30937248]
Level 2 (mid-level) evidence
[8]
Stumbar SE,Stevens M,Feld Z, Cervical Cancer and Its Precursors: A Preventative Approach to Screening, Diagnosis, and Management. Primary care. 2019 Mar;
[PubMed PMID: 30704652]
[9]
Santamaría-Ulloa C, Valverde-Manzanares C. Inequality in the Incidence of Cervical Cancer: Costa Rica 1980-2010. Frontiers in oncology. 2018:8():664. doi: 10.3389/fonc.2018.00664. Epub 2019 Jan 10
[PubMed PMID: 30687639]
Level 2 (mid-level) evidence
[10]
Chrysostomou AC, Stylianou DC, Constantinidou A, Kostrikis LG. Cervical Cancer Screening Programs in Europe: The Transition Towards HPV Vaccination and Population-Based HPV Testing. Viruses. 2018 Dec 19:10(12):. doi: 10.3390/v10120729. Epub 2018 Dec 19
[PubMed PMID: 30572620]