Continuing Education Activity
Fascial dehiscence is a significant complication of abdominal surgery, particularly cases done in an emergent setting. Associated bowel obstructions and strangulation, or evisceration, present a considerable morbidity and mortality burden on the post-operative patient. This activity reviews the etiology and pathophysiology of fascial dehiscence, highlights the evaluation of suspected dehiscence, describes the management of confirmed dehiscence, and identifies interprofessional opportunity to enhance patient outcomes through early detection and intervention.
Objectives:
- Identify the etiology of fascial dehiscence.
- Review the evaluation of wound issues and drainage concerning possible dehiscence in the postoperative patient.
- Describe the operative and nonoperative treatment modalities for patients following fascial dehiscence.
- Summarize interprofessional team strategies for improving care coordination and communication to improve outcomes for patients experience fascial dehiscence.
Introduction
Fascial dehiscence is a concerning complication of open surgical intervention, which often results in the need for additional surgical intervention; dehiscence also represents a significant influence on postoperative morbidity and mortality. High clinical suspicion is essential for early identification and treatment to prevent short- and long-term complications such as chronic wounds, hernias, and evisceration. Optimizing patient factors and surgical techniques can limit the rates of development of fascial dehiscence and thus spare the patient the associated morbidities and need for additional surgical procedures.
Etiology
Traumatic mechanisms, surgical site infections, poor nutrition, comorbidities (i.e., diabetes, chronic steroid use), and prolonged critical care course all increase the risk of fascial dehiscence. Fascial dehiscence places patients at risk of hernias and their associated short- and long-term complications, including obstruction and strangulation. Additionally, progression to evisceration represents a true surgical emergency.[1][2][3][4]
Epidemiology
Fascial dehiscence is a complication of both elective and emergent surgeries. Rates of fascial dehiscence following open elective surgery are 1 to 3%. Emergent operations have a higher rate of fascial dehiscence at 5-50%; when trauma laparotomy is performed, rates from 5-10% for definitive laparotomy. Damage-control procedures whether trauma or emergency general surgery are associated with fascial dehiscence rates of 13 to 50%.[1][2][3][4]
Given the number of open operations performed yearly (approximately 2 million per year in the U.S. from 2009-2013),[5]a notable number of patients have their postoperative course complicated by fascial dehiscence with resultant increases in costs, length of stay, need for additional interventions, and complications.
Pathophysiology
Research has identified several mechanisms for the development of fascial dehiscence, secondary to both modifiable and non-modifiable factors. Traumatic injury requiring exploratory laparotomy is itself a risk factor; presumably, the damage-control nature of the surgery, the open abdomen, and re-exploration (with associated potential for loss of domain), and the often critical nature and course of these patients present likely mechanisms for poor fascial healing. Emergency non-trauma surgical intervention confers a similar risk. The high rates of associated sepsis and intraabdominal contamination, prolonged critical care requirements including the need for vasopressors for shock states, elevated rates of ileus and pulmonary complications, need for planned or unplanned re-exploration and development or continuation of malnutrition states present a myriad of factors influencing the breakdown of the fascial closure.
Surgical site infection is a well-documented cause of dehiscence; bacteria and inflammatory factors present in infected sites are detrimental to wound healing. Nutritional factors, including preoperative malnutrition or prolonged delay in the resumption of postoperative enteral or parenteral nutrition, can deprive the body of needed substrates for adequate healing. Chronic steroid use is a well-known inhibitor of tissue repair; chronic diseases such as ESRD and diabetes and their associated microvascular complications similarly are well-published independent risk factors for failure of wound healing and surgical site infections. Importantly, clinicians cannot ignore a technical error as a cause; loose knots, tissue strangulation, or inadequate tissue incorporation in suture bites can lead to failure of the fascial closure and tissue dehiscence. Smoking is also a well-known inhibitor of wound healing.[6] Obesity has correlations with poor wound healing and skin dehiscence; evidence has not directly linked obesity with fascial dehiscence; however, extrapolation of data suggests a trend in increased fascial complications in obese patients.[7] Seroma/hematoma presents a risk of superficial wound dehiscence, though data are lacking to show a risk of fascial dehiscence.[8]
The physiology of wound healing involves a well-choreographed pathway. Local tissue factors promote platelet aggregation, acute hemostasis, and chemotaxis. The inflammatory response recruits neutrophils, macrophages, and fibroblasts, which clear cellular debris and begin the formation of a regenerative matrix. Angiogenesis occurs, extracellular matrix components get deposited, and collagen forms and remodels. This process is detailed and complicated, requiring multiple cofactors, enzymatic signals, adequate vascularity, and tissue oxygenation. The process commences immediately upon injury, or in this case, surgical incision and closure, and continues for months with remodeling and maturation. Collagen production begins within 12 to 72 hours of injury and peaks within 7 to 21 days; 80% of tissue strength returns at three months. Due to the complicated nature of wound healing, multiple opportunities for factors detrimental to the process are present.
Ultimately, dehiscence results from a failure or inadequacy in the wound healing pathway. Deprivation of tissue repair factors and substrates, overabundance of inflammatory cells and products, presence of clinically significant bacteria load, or malperfusion (from tissue strangulation, microvascular disease, or critical illness) all act at the cellular level to impair the healing pathway; reducing the risk of fascial dehiscence relies upon the optimization of these factors as able given the patient’s clinical status and course.
History and Physical
As with any condition, a thorough history and physical is necessary. The history may be limited in critically ill postoperative patients, particularly if intubated; bedside nurse reports can be essential in this situation. New or markedly increased wound drainage or a new abdominal wall bulging are indicators of dehiscence of the fascia. The clinician should ask the patient about obstructive symptoms, including nausea, vomiting, or obstipation. Patients may report pulling or popping sensation as the inciting symptom; often, this may occur during an episode of coughing, straining, or retching.
The clinician should also elicit surgical history, with a particular focus on recent surgical procedures as dehiscence is most likely during the first 1 to 2 weeks following surgical procedures. The medical history should also have a specific focus on diabetes, conditions requiring chronic steroid use (COPD, adrenal insufficiency, etc.), connective tissue disorders, and malnutrition. Smoking history and an updated medical list are also necessary.
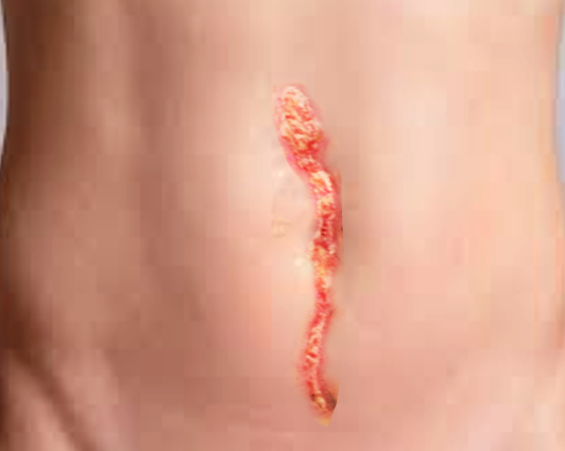
A focused abdominal examination should follow. The prior incision site should undergo inspection for signs of infection, including erythema or drainage. If drainage is present, the character is important; purulent fluid raises suspicion of underlying surgical site infection. Clear or serous-appearing drainage may represent decompressing seroma or may be normal in small quantities as healing progresses. The classic “salmon-colored” drainage is concerning for fascial dehiscence. The abdomen should be palpated to evaluate for any masses or bulging, which could signify seroma, abscess, or herniation of abdominal contents. Peritoneal signs (guarding, rebound tenderness) may be present if organ space infection or bowel ischemia exists.
Often, surgical closures, including staples, sutures, or negative pressure wound therapy devices may be present. If the skin closure remains intact, removal of sutures/staples before imaging or presence of a surgeon is not recommended as opening the incision may lead to the conversion of fascial dehiscence to evisceration requiring emergent surgical intervention. Probing of the wound with sterile swabs (i.e., between staples at areas of persistent skin separation) may be performed to evaluate the integrity of the fascial closure and attempt to detect fascial separation though this is best left to the discretion of a surgeon.
In some instances, patients will present with obvious or suspected dehiscence, as noted during dressing change (packing or negative pressure wound therapy), when superficial tissues have been left open to heal by secondary intention. Fascial defects, with or without visualized underlying bowel loops, may be visible. The diagnosis of fascial dehiscence should be clear in this subset of cases. Bowel evisceration, if noted, should prompt emergent surgical evaluation.
Evaluation
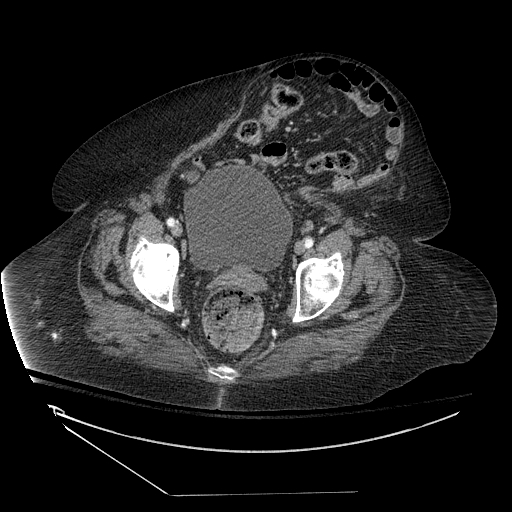
Early involvement of a surgical provider, preferably the surgeon who performed the fascial closure, is prudent for the evaluation and management of suspected dehiscence. In the stable patient with no evidence of evisceration, imaging should be obtained to evaluate for the presence and size of the fascial defect; abdominopelvic CT with contrast provides excellent anatomic details and can also identify additional pathology including superficial/deep/organ space abscesses and herniation of abdominal contents through the fascial defect.
As noted previously, the opening of the incision is not a recommended process, as this can lead to evisceration and the need for an emergent operative procedure. Instead, radiologic evaluation can guide decision-making as to whether elective/urgent operative intervention is necessary.
Treatment / Management
Several clinical factors guide treatment decisions for fascial dehiscence. If a patient experiences evisceration, the bowel should be wrapped in gauze moistened with warm saline to prevent fluid losses, and the patient should be prepared for emergent abdominal exploration to return the bowel to the abdominal cavity and provide overlying closure.
In the stable patient who experiences dehiscence with no evidence of underlying infectious complications, nonoperative management can be considerations if the skin overlying the fascial defect remains closed to prevent evisceration. For patients with a prolonged open abdomen course, abdominal contents may become frozen; these patients are at relatively low risk of bowel herniation and may not require future repair of the defect. For patients who do not have a frozen abdomen, the defect can be monitored and allowed to develop into a controlled hernia, which may be repaired at a future date in an elective fashion once the patient has recovered from the index procedure, and acute inflammation has resolved; importantly, no signs of peritonitis, strangulation, or obstruction should be present if nonoperative management is to merit consideration. Additionally, the clinician must weigh the risk of bowel strangulation posed by small defects.
Indications for operative intervention include evisceration, evidence of bowel strangulation/ischemia, bowel obstruction, or undrained infectious collection. Return of herniated bowel contents to the abdominal cavity should occur. Superficial, deep, or organ space infections should be drained and washed out. Nonviable tissues require debridement; bowel resection may be necessary if bowel segments are irreversibly ischemic.
Multiple techniques are available for closure of the fascial defect. If the dehiscence is small and fascia is healthy, the edges can undergo debridement, and primary closure can take place. Augmentation of the repair with mesh has been shown to improve outcomes and reduce recurrence; surgeons should avoid mesh in infected cases or cases requiring bowel resection, though consideration of biologic mesh repair is reasonable. Large defects that do not easily approximate may need to be managed with progressive wound closure, including open abdomen with vacuum therapy, sequential tightening of progressive closure devices (such as Wittmann patch), or creation of planned hernia via the development of cutaneous flaps closed over the defect. In severe cases, the application of a biologic graft for eventual skin grafting may be necessary.
Abdominal component separation may be useful in some large defects; however, care must be taken not to negatively impact future reconstructive options as patients experience fascial dehiscence requiring reoperation are at high risk of incisional hernia formation in the future. Consequently, component separation in the acute setting is not a routine recommendation. Patients with large defects are likely better treated in the acute setting in a nonoperative manner if able, or with a non-fascial disrupting technique as mentioned above if surgical intervention is needed; staged repair with various component separation techniques can then be pursued in both the expectantly managed groups and those who develop subsequent incisional hernias with greater options for fascial release and closure.[9][10]
If closure does not take place immediately and incisional hernia is allowed to develop, then re-closure can be pursued at a later time to prevent complications related to hernia incarceration, obstruction, or strangulation. Adequate time, usually at least 2 to 3 months, should elapse to allow for healing from the initial procedure. The patient’s clinical status should be optimized, including nutrition factors, in addition to adequate management of comorbidities. Ideally, patients will have returned to their baseline functional status before elective fascial repair. The above-listed techniques are all applicable for re-closure, with the additional benefit of a clean field and the opportunity for non-absorbable mesh placement. No difference has been identified in subsequent wound complications with continuous vs. interrupted suture repair of defects or with the type of suture material used.[11] Mesh repair has been found to have multiple complications, including abscess formation, fistula, adhesive bowel obstruction, and bowel erosion; the markedly decreased recurrence rate for incisional hernia repaired with mesh have been found to outweigh the potential risks of mesh use.[12] Both open and laparoscopic repairs are viable options with low recurrence rates when using a mesh, as opposed to primary fascial repair alone.[12][13]
Differential Diagnosis
Differential diagnosis includes postoperative subcutaneous seroma, which may decompress through the incision with exertion or if it develops substantial size. Surgical site infection with abscess formation is additionally in the differential and may exist concurrently with fascial dehiscence given the association between infection and fascial closure breakdown. Pre-existing hernias may also be present and may not have been addressed in the index operation, so abdominal bulging away from the incision may represent hernia, which could be pre-existing or enlarging.
Prognosis
Fascial dehiscence after abdominal surgery is a major contributor to patient morbidity and mortality. Subsequent complications following dehiscence are estimated to be as high as 75%; published reports on mortality range from 15 to 50%. Prolonged hospital course (15 to 26 days), increased intensive care requirements, and higher healthcare costs are all associated with abdominal wall complications following laparotomy. While prompt recognition and treatment are important, the development of fascial dehiscence presents a substantial morbidity and mortality burden for the patient irrespective of the timing of subsequent intervention.[14][15][16]
Complications
As noted above, the development of fascial dehiscence places a considerable morbidity and mortality burden on the affected patient. Development of subsequent infections is a concern with an approximate 10% risk; abdominal washout should be a consideration, and skin/subcutaneous tissues may be left open to heal by secondary intention when urgent/emergent reoperation is necessary, and the surgeon encounters frankly contaminated, or necrotic tissue.[17] Complications include death, evisceration, and associated infections in the acute setting; bowel herniation can occur, and an incisional hernia can form with the development of subsequent bowel obstruction and/or strangulation in both the short- and long-term setting. The need for additional procedures to repair the defect or hernia, reduce or resect involved bowel, and drain accumulated fluid collections potentiate the risks of anesthesia-, surgical- and hospital-associated complications including pneumonia, hospital-acquired infection, prolonged ICU courses, deconditioning, adhesive small bowel obstruction, venous thromboembolic events, and need for short- or long-term rehab/nursing care. Notably, fascial dehiscence can vastly impact a patient’s duration, and quality of life and consequently modifiable risk factors (both patient and technical) should be managed to improve patient outcomes.[14][15][16]
Deterrence and Patient Education
Multiple modifiable factors exist which can alter the outcome of abdominal surgeries and can influence the prevention (or formation) of wound complications, including fascial dehiscence. For the elective patient setting, smoking cessation before surgical intervention is optimal.[18][19] Blood glucose should be under control in the pre-, peri- and postoperative setting.[20] Steroid use should be avoided or minimized as able; however, this may not be feasible in patients on chronic steroids. Nutrition requires supplementation and nutritional parameters may be measured if concern for preoperative malnutrition exists. Intraabdominal distention secondary to ileus, bowel obstruction, or urinary retention should be managed with decompression (i.e., nasogastric tube placement, catheterization). Pulmonary interventions to reduce atelectasis and promote oxygenation should be implemented.
For the damage-control or open abdomen patients, closure of the abdomen as soon as clinically safe is the recommended course; more than four reoperations before closure has been found to be associated with a significant increase in the rates of failure of primary fascial closure.[21] Delayed closure in the setting of resuscitative efforts can result in bowel edema, which increases intraabdominal pressure upon closure of the fascia. Loss of abdominal wall domain can occur, and fascial edges may not be approximated with minimal amounts of tension, thus increasing the risk for a breakdown of the closure. In the setting of loss of domain, multiple strategies exist to aid in closure including botulinum toxin, sequential closure with hook-and-loop devices, component separation, progressive pneumoperitoneum, and mesh (typically biologic) utilization; in severe cases, skin flaps may be raised and closed without fascial re-approximation creating a controlled hernia.[22][23]
Incision type has been considered to be a risk factor for the development of dehiscence. Data is not strong; however, trends towards a lower rate of fascial complications have occurred for transverse or oblique incisions as opposed to the midline or paramedian incisions.[24] However, the type of incision appropriate for the planned procedure often dictates the decision regarding incision placement and orientation.
For all fascial closures, attention to the surgical technique is essential. Care should be taken to snugly approximate fascial edges without strangulating tissue. Knots should be securely tied. Monofilament suture has been found to be associated with lower rates of subsequent dehiscence as compared to multifilament suture.[25] No difference in dehiscence rates has been identified with the use of absorbable vs. non-absorbable suture. Additionally, no difference in dehiscence rates has correlated to continuous vs. interrupted sutures or mass vs. layered fascial closure.[25][26][27] Weak evidence suggests that a “small-bites” technique, with 5-8 mm fascial bites placed every 5 mm, may reduce the incidence of complications.[28] The closure (or non-closure) of the peritoneal layer is not associated with the development of fascial dehiscence.[29][27] Evidence suggests that a suture to wound length ratio of 4:1 in continuous suture closure reduces fascial closure breakdown.[30] Drains have not been found to prevent fascial dehiscence, however drains or ostomies should not be sited within the midline incision as these can increase the incidence of fascial problems. [29]
The use of retention sutures (internal or external) is a long-standing topic of discussion in the surgical realm, particularly in terms of fascial dehiscence and evisceration. Classic teaching states that retention sutures prevent evisceration, however, do not serve to prevent fascial dehiscence; a recent small randomized series contradicts this finding with a decrease in dehiscence rates noted in the retention suture group.[31] Ultimately, retention suture use is left to the discretion of the operating surgeon, though likely only presents a benefit in patients at high risk of wound complications.[31]
Patients should be educated on wound care and concerning findings. Many surgeons will implement activity restrictions following abdominal surgery to prevent failure of the fascial closure. These tend to vary in length by surgeon and procedure. No strong evidence exists to support these recommendations as unavoidable activities of daily living (i.e., getting up from chair, coughing, straining with Valsalva) may equal or exceed the intraabdominal pressures generated with lifting;[32] nevertheless, counseling patients to avoid straining, heavy lifting, and activities that cause incisional pain for several weeks following surgery is likely prudent. Patients should also receive instruction regarding concerning features that could develop including increasing or large volume wound drainage, development of focal bulging, or evidence of wound infection; early notification of their primary surgeon and clinical evaluation (in the office or emergency department) may prevent subsequent development of more serious wound complications.
Enhancing Healthcare Team Outcomes
An interprofessional approach to patient care can act to prevent the development of fascial dehiscence and to enhance the recovery if it occurs via early recognition and intervention. Wound-care specialized nursing and other wound care clinicians provide a first-line opportunity to minimize risk via detection of increasing abdominal distention (i.e., ileus, obstruction) in need of decompression, urinary retention requiring catheterization, respiratory complications necessitating treatment, or wound drainage (either new-onset or with a change in character). Pharmacy involvement will come in the form of antimicrobial interventions if necessary; the pharmacist can verify dosing, perform medication reconciliation, and report any concerns to the medical team. Additionally, bedside wound care presents an opportunity for evaluation of wound healing and notice of developing issues. Respiratory care can provide benefit with pulmonary hygiene treatments to minimize atelectasis and promote oxygenation.
Also, the outpatient or emergency department providers may encounter with a patient experiencing fascial dehiscence as patients often seek initial care at their primary care office or in the emergency room. A high index of suspicion and early referral or consultation of a surgical provider offers an opportunity for optimum patient care.
Interprofessional collaboration and communication are essential team elements in the care and management of fascial dehiscence patients, leading to improved outcomes. [Level 5]