Continuing Education Activity
Transcatheter closure of the ASD is currently available for secundum type of ASDs, and currently, there are two FDA-approved devices in the United States for the closure of ASD. This activity reviews the technique of ASD closure, indications, contraindications and highlights the interprofessional team's role in managing patients with congenital heart defects.
Objectives:
- Identify the technique of catheter management of ASD.
- Describe the indications for catheter management of ASD.
- Recall the complications of catheter management of ASD.
- To improve outcomes, discuss the importance of interprofessional team care when managing patients undergoing ASD closure with a catheter.
Introduction
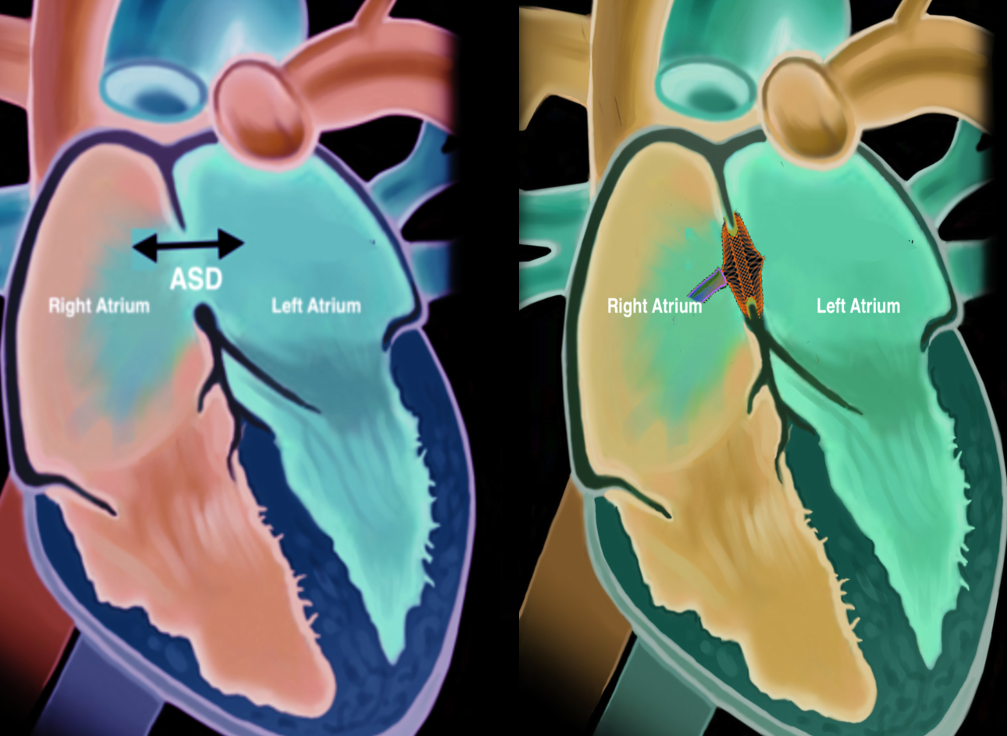
Atrial septal defect (ASD) is one of the most common congenital heart defects with an estimated incidence of 6 to 10 per 10,000 live births.[1] Atrial septal defects are classified as primum, secundum, sinus venosus, and coronary sinus defects. The onset of symptoms varies among different patients, and many patients remain asymptomatic. Larger defects tend to cause symptoms early on, and smaller defects cause symptoms later in life. Symptoms include failure to thrive, shortness of breath, palpitations, enlargement of right heart chambers, peripheral edema, cyanosis, orthodeoxia-platypnea, and paradoxical embolism. Transcatheter closure of the ASD is currently available for secundum type of ASDs, and currently, there are two FDA-approved devices in the United States for the closure of ASD.
Anatomy and Physiology
The heart comprises four chambers; the two upper chambers are referred to as the atria, and the two lower chambers are referred to as the ventricles. The inter-atrial septum divides the atrium into the right and the left atria. Similarly, a septum separates the ventricles into the right and left and is called the inter-ventricular septum. Embryologically the inter-atrial septum is derived from the septum primum and the septum secundum. The septum primum arises from the roof of the atrium and develops towards the endocardial cushions covering the ostium primum. This is followed by degeneration of the septum primum towards the roof of the atria creating the ostium secundum. Next, the septum secundum arises from the atrial roof on the right atrial side and grows caudally to cover the ostium secundum.[2] Depending on the location of the defect, ASDs are classified into primum, secundum, sinus venosus, and coronary sinus defects. Defects of the inter-atrial septum at the ostium secundum (fossa ovalis) are referred to as secundum type ASD, which is the most common type of ASD. The primum defects involve the ostium primum and the endocardial cushions and are associated with atrioventricular valvular abnormalities. The sinus venosus defects are located at the junction of the superior or inferior vena cava with the right atrium and are called superior and inferior sinus venosus defects in relation to the superior or inferior vena cava. The superior sinus venosus defects are associated with anomalous pulmonary vein connections. In the coronary sinus defect, there is a loss of the roof of the coronary sinus that allows for direct communication between the left atrium and the coronary sinus.[3] Based on the size, ASDs are classified into[4]:
- Trivial: Less than 3 mm in diameter
- Small: 3 to less than 6 mm in diameter
- Moderate: 6 to 8 mm in diameter
- Large: Greater than 8 mm in diameter[4]
Indications
Transcatheter closure of the ASD can be performed for only the secundum type of ASDs. The other ASD types are repaired surgically because of their location and associated abnormalities of atrioventricular valve defects (primum type) and anomalous pulmonary vein connections (sinus venosus type).
Current indications for ASD closure by a transcatheter approach include:
- Symptomatic ASD causing functional impairment (Class I)
- Symptomatic (Class I) or asymptomatic right atrial and or right ventricular enlargement (Class II)
- Symptomatic (Class I) of the asymptomatic hemodynamically significant defect without symptoms (Ratio of pulmonary to systemic flow greater than 1.5) (class II)
- Paradoxical embolism[5][6][7]
Contraindications
Contraindications for ASD closure by a transcatheter approach include:
- ASDs other than those of the secundum type, including primum type, sinus venosus type, and coronary sinus defects
- Severe pulmonary hypertension (pulmonary systolic pressure or pulmonary vascular resistance greater than two-thirds of systemic pressure or systemic vascular resistance)
- Eisenmenger syndrome or net right to left shunt
- Defects larger than 38 mm in diameter
- Absent or insufficient rim of tissue around the defect[5][7]
Personnel
Interventional cardiologist trained and experienced in structural heart disease, cardiovascular cath laboratory nurse, first assistant, and cardiac cath laboratory technician.
Preparation
Pre-Procedure Evaluation
The patient should be evaluated clinically and through history, including any allergies should be assessed especially to metals such as nickel. The patient should undergo a transesophageal echocardiogram to evaluate the type of ASD and its suitability for repair, such as assessing the rims of tissue around the ASD. The defect size should be measured to size the device. The patient should also undergo a right heart catheterization to assess the pulmonary pressures and the shunt fraction. Cardiac magnetic resonance imaging or cardiac CT angiography is recommended to assess the defect and anomalous pulmonary vein connections if this cannot be assessed during the transesophageal echocardiogram.
Once eligibility is determined, the patient should be counseled about the risks and benefits of undergoing the procedure, and consent should be obtained. On the day of the procedure, the patient should be evaluated for any active ongoing infections and determine suitability for anesthesia.
Technique or Treatment
Transcatheter closure of ASD is performed under moderate sedation or general anesthesia depending on the complexity of the defect and the need for TEE imaging. But the vast majority of the ASD defects can be closed under moderate sedation. A preoperative antibiotic is administered before the procedure. After sedating the patient, vascular access is obtained in the femoral vein. Depending on the operator's preference, venous access can be obtained on the right femoral vein, or bilateral femoral veins can be used. Two accesses are required to perform the procedure if using ICE for imaging. If TEE is used, then a single venous access is adequate. Access for the ICE catheter requires an 8-10 French sheath placement and preferably a length of 25-35 cm sheaths are used as this gives stability to the ICE catheter and helps avoid the tortuosity of the femoral veins. The second access, depending on the operator preference, can start with a 6 French and can be upgraded to the appropriate sheath size in the range of 6-12 French depending on the size of the closure device being used. The higher size sheaths are required for delivering larger sized devices. Additional arterial access may be obtained for pressure monitoring, depending on the operator. Once both accesses are obtained, the patient is anticoagulated, preferably with unfractionated heparin, but other agents such as bivalirudin can be used for patients with heparin allergy. ACT of > 250 is maintained for the duration of the procedure.
Next, the ICE catheter is advanced under fluoroscopic guidance into the right atrium and positioned to visualize the interatrial septum. Thereafter the septum is then visualized, and the interatrial septum is interrogated for the presence of appropriate rim size. The ASD size is measured in multiple planes, and color flow and the direction of the shunt are recorded. After this, an angled tip catheter is advanced over a J-tip guidewire into the right atrium. The defect is crossed with the J-tip guidewire from the right atrium into the left atrium under fluoroscopic and ICE guidance. Thereafter the J-tip guidewire is advanced into the left superior pulmonary vein, and the catheter is advanced into the left superior pulmonary vein. The J-tip guidewire is then exchanged for a super stiff guidewire, and the angled tip catheter is removed. After this, the venous sheath in the femoral vein is removed, and an ASD sizing balloon is advanced over the wire into the ASD. Under fluoroscopic guidance, the sizing balloon is slowly inflated with contrast until the flow across the ASD stops as visualized by the ICE. At this, care should be taken to avoid overinflation of the balloon as this will overestimate the size of the defect. The preferable approach is to deflate the balloon until the flow across the ASD resumes and thereafter inflate the balloon until the flow across the ASD ceases again; this is called the "stop-flow" technique. Next, the size of the defect will be measured on both fluoroscopy and the ICE using the indentation marks on the ASD sizing balloon. After this, the balloon is deflated and removed over the wire with care taken to prevent the wire from prolapsing out from the pulmonary vein. Following this, the appropriately sized sheath that can deliver the ASD device is advanced over the wire into the left atrium, and the sheath dilator and the wire are removed. A stopcock or a syringe should be attached to the sheath to prevent inadvertent air entry into the sheath.
Next, the appropriately sized ASD device is prepped. The device is first checked for integrity, and the device is immersed in a saline tub. After this, the device is attached to the delivery cable, and the device is slowly pulled back into the loader sheath while the side flush of the loader sheath is constantly flushed to prevent any air from being trapped in the device or the loader sheath. Next, the loader is attached to the delivery sheath carefully without the introduction of air. The delivery cable is advanced into the sheath, thereby advancing the device into the delivery sheath. Thereafter, the device is slowly advanced towards the tip of the sheath under fluoroscopic guidance. At this point, care should be taken to reposition the ICE catheter to optimize the visualization of the ASD as sometimes the ICE catheter moves while the above steps are being performed. Once the device reaches the tip of the sheath, then the sheath is withdrawn over the delivery cable to unsheath the left atrial disc. This can be visualized on both ICE and fluoroscopy. Once the left atrial disc is fully formed, both the delivery sheath and the delivery cable are withdrawn until the left atrial disc is well apposed to the interatrial septum on the left atrial side. A little tension should be maintained to cause tenting of the interatrial septum. Excess tension on the sheath or the delivery cable during this step may cause the left atrial disc to prolapse into the right atrium, in which case the left atrial disc should be withdrawn into the sheath. The device should be removed, and the entire steps from crossing the septum to the advancement of the sheath should be repeated.
Once the left atrial disc anchors well on the left side of the septum and there are sufficient tension on the interatrial septum by the left atrial disc, the next step would be to unsheath the right atrial disc carefully. After this, the sheath is slightly withdrawn away from the right atrial disc to decrease tension on the device. After this, ICE is used to examine the stability of the device, and color doppler imaging is performed to verify that the ASD is well sealed. Color flow may be noted across the waist and the center of the device, but color flow at the edges of the discs or away from the disc suggests that the device may be smaller in size, or there may be additional ASD's away from the current ASD location. In either case, the device should be captured back into the sheath, and the septum examined again for the presence of other ASD's in proximity. In this situation, the device can be upgraded to a bigger sized ASD device or cribriform occluder if the other ASD's are close to the primary ASD. If the other ASD's are > 7 mm from the primary ASD, then the other defects need to be closed using additional ASD occluders. If there is no color flow across the device, then the delivery cable is wiggled with a strong push and pull (Minnesota Wiggle) to examine the stability of the device by both fluoroscopy and ICE. If the device looks stable and there is no flow across the device, the device is released. Once the device is released, the stability and color flow are again checked, and if they are satisfactory, then the sheath is withdrawn into the inferior vena cava. Following this, the ICE imaging is performed to visualize the device and look for pericardial effusion. If everything looks satisfactory, then the ICE catheter is withdrawn from the body. Next, both the femoral venous sheath and the delivery sheath are withdrawn from the body, and either a figure of 8 stitch is placed, or manual pressure is held to achieve hemostasis. Protamine may be given to reverse the anticoagulation at this time, depending on operator preference.
The patient is given clopidogrel 300-600 mg orally in addition to aspirin, and dual antiplatelet therapy is continued for 6 months after the procedure. After the procedure, the patient is monitored overnight. A follow-up echocardiogram is performed the following day to verify the stability of the device and rule out any erosion from the device and rule out any pericardial effusion. If everything is satisfactory, then the patient is discharged, and the patient is followed up in 6 months with a repeat echocardiogram. Infective endocarditis prophylaxis is recommended for 6 months for dental procedures.[9][10]
Complications
Major complications related to the transcatheter ASD closure include:
- Device embolization
- Erosion of the cardiac structures from the device
- Atrial arrhythmias
- Atrioventricular block (AV block)
- Persistent atrial aneurysm
- Thromboembolism
- Pericardial effusion and tamponade[11][12][13]
Clinical Significance
Current guidelines recommend either surgery or transcatheter closure of the secundum ASD. However, transcatheter closure of the ASD is a relatively safe and effective procedure for the closure of the ASD in appropriately selected patients and is associated with low morbidity. Therefore, transcatheter closure has become the choice of therapy for the closure of secundum ASD defects.
Enhancing Healthcare Team Outcomes
Transcatheter ASD closure is a relatively safe procedure with a low complication rate and faster recovery. An interprofessional heart team approach is recommended for a thorough evaluation of the patient by a cardiologist and an interventional cardiologist experienced in treating structural heart disease to assess the appropriate treatment strategy for every patient. Typically, these procedures should be performed at high-volume centers to obtain optimal outcomes.