Continuing Education Activity
Cryptococcus is an encapsulated fungus and an opportunistic pathogen. These commonly infect the central nervous system but can present with a variety of skin manifestations such as a papule, a maculopapular lesion or a violaceous nodular lesion. This activity reviews the evaluation and treatment of cutaneous cryptococcosis and explains the role of the interprofessional team members in managing patients with this condition.
Objectives:
- Summarize the epidemiology of cutaneous cryptococcosis.
- Review the pathophysiology of cutaneous cryptococcosis.
- Describe the use of biopsy in the evaluation of cutaneous cryptococcosis.
- Outline the importance of improving care coordination among the interprofessional team to improve outcomes for patients with cutaneous cryptococcosis.
Introduction
Cryptococcus is an encapsulated hetero-basidiomycetous fungus that is an opportunistic pathogen. These fungi are present in the environment in sexual and asexual forms. These fungi infect immunocompromised host as well as immunocompetent persons. These fungi show a strong tropism for the central nervous system but are also known for their dermatographism.[1][2][3][4]
Etiology
Cryptococcus was first identified in peach juice in Italy by Sanfelice in 1894. In 1895, the first human case was reported. This case was a young woman with a chronic, non-healing skin ulcer on her shin. Yeasts were found in her ulcer, and later at autopsy, yeasts were found in multiple internal organs. These yeasts were Cryptococcus neoformans. The sexual form of this fungus was identified in 1976 and named Filobasidiella neoformans. Thus far, 19 species of Cryptococcus have been identified. Two are pathogenic. There are two serotypes in each of these species. Cryptococcus neoformans var. neoformans is serotype D, Cryptococcus neoformans var. grubii is serotype A, and Cryptococcus gattii has two serotypes: B and C. The Cryptococcus neoformans genome was published in 2005.[5][6][7]
Cryptococcus species are found in soil contaminated by guano. Cryptococcus gattii has a clear association with eucalyptus trees and a variety of coniferous trees.
The life cycle of this fungus involves two distinct forms, sexual and asexual. The asexual form exists as yeast that divides by narrow-based budding. Yeast forms are primarily seen in clinical specimens. The sexual forms exist in nature in two mating types, “alpha” or “a.” When opposite types mate, meiosis occurs, resulting in chains of basidiospores. These basidiospores are 1 micrometer to 2 micrometer long and are the infectious particles, which are inhaled and reach the alveoli.
Cryptococcus grows well on fungal or bacterial culture mediums in 48 to 72 hours. The colonies appear mucoid.
Epidemiology
Cryptococcus neoformans is endemic in the United States along the Pacific coast extending into Canada. Cryptococcus gattii was recently reported from northern California and Vancouver Island in Canada. C. gattii is endemic in Papua, New Guinea, and northern Australia. While C. neoformans mainly affects patients with AIDS and those who are immunosuppressed, a quarter of patients with C. gattii infections are immunocompetent and healthy.
Prior to the AIDS epidemic, the incidence of Cryptococcus disease was very low at 0.8 per million in the United States, but in 1992 at the height of AIDS epidemic, the incidence increased to 5 per 100,000. Since the use of ART, the incidence has declined to very low levels once again. However, in sub-Saharan Africa, where the AIDS epidemic is raging, the burden of Cryptococcus disease is very high. In sub-Saharan Africa, cryptococcal meningitis is the most common culture-positive meningitis and leads to half a million deaths annually, which is more than mortality due to tuberculosis. Therefore, this disease is a marker of HIV patients with poor access to health care.
Most patients with disseminated disease have an underlying immunocompromised state such as AIDS, transplant patients, those on long-term corticosteroids and those who are prescribed monoclonal antibodies.
Pathophysiology
The polysaccharide capsule, production of melanin, and growth at high temperatures are considered virulence factors for Cryptococcus species. The initial exposure occurs via the lungs through inhalation of basidiospores (1 micrometer to 2 micrometer in length) or dehydrated yeast of appropriate size. Most immunocompetent patients do not succumb to disease unless the inoculum is overwhelming. An effective immune response is generated by alveolar macrophages, which eliminate the yeast. In immunocompromised patients, the yeast continues to divide within the macrophages, which disseminate the infection throughout the body. Skin is the third most common organ system to be involved after lungs and the central nervous system.
History and Physical
Cryptococcus infections present with a wide variety of skin lesions. Skin lesions are frequently a sentinel for disseminated disease; however, primary cutaneous lesions do occur in immunocompetent persons. The primary cutaneous lesion may be a papule, maculopapular lesion with an ulcerated center or a violaceous nodular lesion. Often there is a discharging sinus that connects to the underlying deep abscess or the underlying bone. Some strains of C. neoformans show dermatropism. However, primary cutaneous cryptococcosis is a diagnosis of exclusion.
Skin lesions in HIV patients can be identical to a bacterial abscess in appearance and clinical presentation. Cutaneous cryptococcosis may present as a cold abscess, without fever and signs of inflammation. Therefore, it is important to send cultures from any abscess drained from an immunocompromised patient. Cutaneous cryptococcal lesions in an AIDS patient may present as crusty, ulcerative lesions that develop slowly. Sometimes the skin lesions of Cryptococcus are umbilicated and can be mistaken for Molluscum contagiosum. Frequently there is simultaneous pulmonary and central nervous system (CNS) involvement.
Cryptococcosis is the third most common invasive fungal infection in organ transplant recipients, after candidiasis and aspergillosis. It is often a late disease occurring at three to ten years post-transplant. Solid organ transplant (SOT) patients have a greater propensity for Cryptococcus skin infections when compared to hematopoietic stem cell transplant patients, for unclear reasons. Cutaneous cryptococcosis can mimic acute bacterial cellulitis in solid organ transplant patients. Cutaneous manifestations can be protean, may mimic an abscess, present as cellulitis that later ulcerates, blisters and shows necrosis, or mimic panniculitis. Primary cutaneous lesions do occur in solid organ transplant patients, but a disseminated disease is more common. In a study of 146 patients with cutaneous cryptococcosis, the following lesions were present in one-third of the patients: nodular mass, papule, ulcer/abscess, and cellulitis. Two-thirds of the skin lesions occurred in the lower extremities, and in 70% of cases, the patients had disseminated disease. The CNS was involved in 90% of cases with disseminated disease.
Evaluation
No skin lesion is characteristic of cutaneous cryptococcosis; therefore, a biopsy is important. A good epidemiological history is important. Patients must be evaluated for an underlying immunosuppressed state. Patients with cutaneous cryptococcosis should be evaluated for the involvement of other organ systems such as the lungs and the CNS.[8][9]
The polysaccharide capsule antigen is a large molecule, and assays have been developed to detect the antigen in serum and other body fluids. If cryptococcal polysaccharide antigen (CA) is present in cerebrospinal fluid (CSF), then there is a good chance that the yeast is present as well. The cryptococcal polysaccharide antigen assay is nearly 100% sensitive and 96% to 99.5% specific in serum and 96% to 100% sensitive and 93.5% to 99.8% specific in CSF.
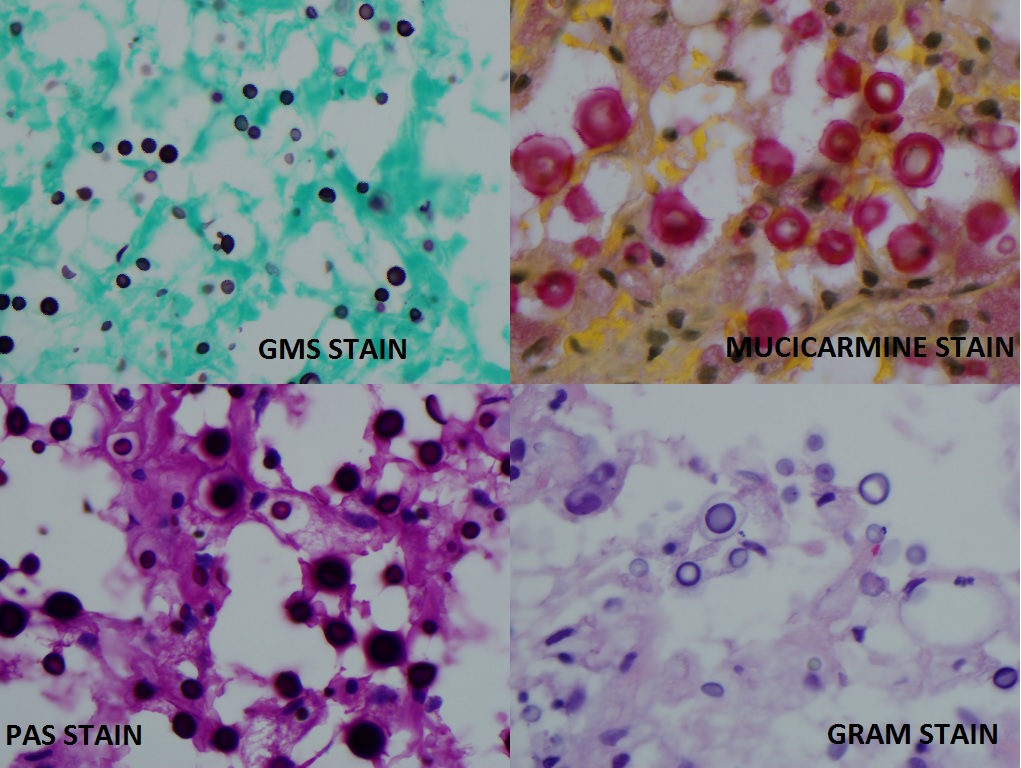
Other microbiological tests to identify Cryptococcus are India-ink preparations and Grocott's methenamine silver stain (GMS). Cryptococcemia can be easily detected by automated systems and seldom results in shock or signs and symptoms of sepsis.
Treatment / Management
Treat HIV patients with a disseminated cryptococcal disease with Amphotericin B (0.7 mg/kg/d to 1.0 mg/kg/d intravenously (IV)) and flucytosine (100 mg/kg/d to 125 mg/kg/d divided into four oral doses) for two weeks, which is the induction phase. Follow this therapy with fluconazole (400 mg [6 mg/kg] per day orally) for a minimum of eight weeks which is the consolidation phase. All forms of amphotericin preparations work equally well, but liposomal amphotericin (AmB) may be preferred in the setting of renal dysfunction. Fluconazole 200 mg daily by mouth is then used as suppressive or maintenance therapy. The duration of maintenance therapy can extend from six months to two years. If the patient is on antiretroviral therapy (ART), then it is reasonable to stop suppression after 12 months of therapy when the viral load is undetectable, and CD4 count is over 100 for three months.
In organ transplant patients with disseminated disease, the regimen is the same, although liposomal amphotericin (AmB) (3 mg/kg/d to 4 mg/kg/d IV) may be preferred. If there is a large fungal burden, a higher dose of AmB (6 mg/kg/d) should be used. If flucytosine is not available for use in the induction phase, then amphotericin therapy should be extended to four weeks. After that, fluconazole maintenance therapy should be continued for at least six to 12 months. Immunosuppressive management should include gradual withdrawal of immunosuppressants, beginning with corticosteroids.
In immunocompetent patients, the principles of therapy are the same. Begin induction therapy with AmB (0.7 mg/kg to 1.0 mg/kg per day IV) plus flucytosine (100 mg/kg per day orally in four divided doses) for at least four weeks. Follow this with eight weeks of consolidation therapy with fluconazole (800 mg [12 mg/kg] per day orally) for eight weeks. After that, continue maintenance therapy with fluconazole (200 mg [3 mg/kg] per day orally) for six to 12 months.
Differential Diagnosis
- Acute complications of sarcoidosis
- Atypical mycobacterial diseases
- Dermatological manifestations of Coccidioidomycosis
- Dermatological manifestations of Kaposi sarcoma
Enhancing Healthcare Team Outcomes
The diagnosis and management of disseminated cryptococcal disease is with an interprofessional team that includes an infectious disease expert, internist, primary care provider, neurologist, pulmonologist and dermatologist. The majority of these patients are immunosuppressed and thus therapy is often extended for several years. The outlook for these patients is guarded and depends on the cause of immunosuppression, extent and other comorbidity. [10](Level V)