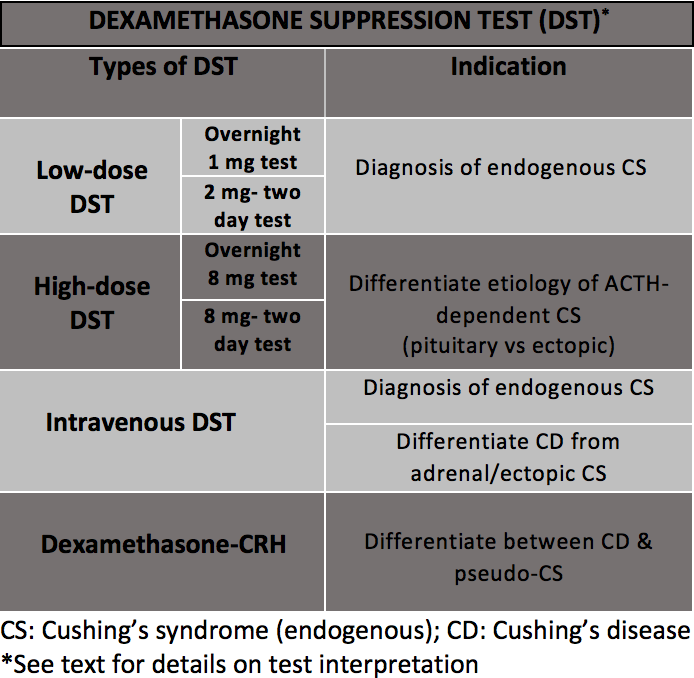
[1]
Keevil BG. Improving the Dexamethasone Suppression Test. Clinical chemistry. 2021 Jul 6:67(7):929-931. doi: 10.1093/clinchem/hvab076. Epub
[PubMed PMID: 34125167]
[2]
LIDDLE GW. Tests of pituitary-adrenal suppressibility in the diagnosis of Cushing's syndrome. The Journal of clinical endocrinology and metabolism. 1960 Dec:20():1539-60
[PubMed PMID: 13761950]
[3]
Williams DM. Clinical Pharmacology of Corticosteroids. Respiratory care. 2018 Jun:63(6):655-670. doi: 10.4187/respcare.06314. Epub
[PubMed PMID: 29794202]
[4]
Tanaka K, Toriumi M, Itoh S, Ogino Y. [Dexamethasone suppression test]. Nihon rinsho. Japanese journal of clinical medicine. 1997 Apr:55 Suppl 2():353-6
[PubMed PMID: 9172546]
[5]
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing's syndrome: an Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. 2008 May:93(5):1526-40. doi: 10.1210/jc.2008-0125. Epub 2008 Mar 11
[PubMed PMID: 18334580]
Level 1 (high-level) evidence
[6]
Fleseriu M, Auchus R, Bancos I, Ben-Shlomo A, Bertherat J, Biermasz NR, Boguszewski CL, Bronstein MD, Buchfelder M, Carmichael JD, Casanueva FF, Castinetti F, Chanson P, Findling J, Gadelha M, Geer EB, Giustina A, Grossman A, Gurnell M, Ho K, Ioachimescu AG, Kaiser UB, Karavitaki N, Katznelson L, Kelly DF, Lacroix A, McCormack A, Melmed S, Molitch M, Mortini P, Newell-Price J, Nieman L, Pereira AM, Petersenn S, Pivonello R, Raff H, Reincke M, Salvatori R, Scaroni C, Shimon I, Stratakis CA, Swearingen B, Tabarin A, Takahashi Y, Theodoropoulou M, Tsagarakis S, Valassi E, Varlamov EV, Vila G, Wass J, Webb SM, Zatelli MC, Biller BMK. Consensus on diagnosis and management of Cushing's disease: a guideline update. The lancet. Diabetes & endocrinology. 2021 Dec:9(12):847-875. doi: 10.1016/S2213-8587(21)00235-7. Epub 2021 Oct 20
[PubMed PMID: 34687601]
Level 3 (low-level) evidence
[7]
Spencer RL, Deak T. A users guide to HPA axis research. Physiology & behavior. 2017 Sep 1:178():43-65. doi: 10.1016/j.physbeh.2016.11.014. Epub 2016 Nov 18
[PubMed PMID: 27871862]
[8]
Aguilera G, Liu Y. The molecular physiology of CRH neurons. Frontiers in neuroendocrinology. 2012 Jan:33(1):67-84. doi: 10.1016/j.yfrne.2011.08.002. Epub 2011 Aug 18
[PubMed PMID: 21871477]
[9]
Brownstein MJ. Adrenocorticotropic hormone (ACTH) in the central nervous system. Advances in biochemical psychopharmacology. 1980:22():93-9
[PubMed PMID: 6249088]
Level 3 (low-level) evidence
[10]
Tanaka K. [Cortisol]. Nihon rinsho. Japanese journal of clinical medicine. 1995 Mar:53 Su Pt 2():437-40
[PubMed PMID: 8753275]
[11]
Hodes A, Meyer J, Lodish MB, Stratakis CA, Zilbermint M. Mini-review of hair cortisol concentration for evaluation of Cushing syndrome. Expert review of endocrinology & metabolism. 2018 Sep:13(5):225-231. doi: 10.1080/17446651.2018.1517043. Epub 2018 Sep 20
[PubMed PMID: 30234410]
[12]
Sederberg-Olsen P, Binder C, Kehlet H. Urinary excretion of free cortisol in impaired renal function. Acta endocrinologica. 1975 Jan:78(1):86-90
[PubMed PMID: 1172895]
[13]
Billon-Rey S, Beylot M, Mathian B, Patricot MC, Berthezene F, Mornex R, Revol A. [Comparison between the value of urinary free cortisol and 17-hydroxycorticosteroids for the diagnosis of Cushing's syndrome]. Presse medicale (Paris, France : 1983). 1986 May 24:15(21):965-8
[PubMed PMID: 2942852]
[14]
Mengden T, Hubmann P, Müller J, Greminger P, Vetter W. Urinary free cortisol versus 17-hydroxycorticosteroids: a comparative study of their diagnostic value in Cushing's syndrome. The Clinical investigator. 1992 Jul:70(7):545-8
[PubMed PMID: 1327327]
Level 2 (mid-level) evidence
[15]
Lee DY, Kim E, Choi MH. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB reports. 2015 Apr:48(4):209-16
[PubMed PMID: 25560699]
[16]
Lowrance SA, Ionadi A, McKay E, Douglas X, Johnson JD. Sympathetic nervous system contributes to enhanced corticosterone levels following chronic stress. Psychoneuroendocrinology. 2016 Jun:68():163-70. doi: 10.1016/j.psyneuen.2016.02.027. Epub 2016 Feb 26
[PubMed PMID: 26974501]
[17]
Maidana P, Bruno OD, Mesch V. [A critical analysis of cortisol measurements: an update]. Medicina. 2013:73(6):579-84
[PubMed PMID: 24356273]
[18]
Gatti R, Antonelli G, Prearo M, Spinella P, Cappellin E, De Palo EF. Cortisol assays and diagnostic laboratory procedures in human biological fluids. Clinical biochemistry. 2009 Aug:42(12):1205-17. doi: 10.1016/j.clinbiochem.2009.04.011. Epub 2009 May 4
[PubMed PMID: 19414006]
[19]
El-Farhan N, Rees DA, Evans C. Measuring cortisol in serum, urine and saliva - are our assays good enough? Annals of clinical biochemistry. 2017 May:54(3):308-322. doi: 10.1177/0004563216687335. Epub 2017 Mar 16
[PubMed PMID: 28068807]
[20]
Rosmalen JG, Kema IP, Wüst S, van der Ley C, Visser ST, Snieder H, Bakker SJ. 24 h urinary free cortisol in large-scale epidemiological studies: short-term and long-term stability and sources of variability. Psychoneuroendocrinology. 2014 Sep:47():10-6. doi: 10.1016/j.psyneuen.2014.04.018. Epub 2014 May 4
[PubMed PMID: 25001951]
Level 2 (mid-level) evidence
[21]
Salazar-García S, Lares-Villaseñor E, Bárcenas-Morales A, Vargas-Morales Juan M. Impact of chemical preservative in urine samples. EJIFCC. 2020 Mar:31(1):56-64
[PubMed PMID: 32256289]
[22]
Poulsen CS, Kaas RS, Aarestrup FM, Pamp SJ. Standard Sample Storage Conditions Have an Impact on Inferred Microbiome Composition and Antimicrobial Resistance Patterns. Microbiology spectrum. 2021 Oct 31:9(2):e0138721. doi: 10.1128/Spectrum.01387-21. Epub 2021 Oct 6
[PubMed PMID: 34612701]
[23]
Turpeinen U, Hämäläinen E. Determination of cortisol in serum, saliva and urine. Best practice & research. Clinical endocrinology & metabolism. 2013 Dec:27(6):795-801. doi: 10.1016/j.beem.2013.10.008. Epub 2013 Oct 25
[PubMed PMID: 24275191]
[24]
Casals G, Hanzu FA. Cortisol Measurements in Cushing's Syndrome: Immunoassay or Mass Spectrometry? Annals of laboratory medicine. 2020 Jul:40(4):285-296. doi: 10.3343/alm.2020.40.4.285. Epub
[PubMed PMID: 32067427]
[25]
Alwani RA, Schmit Jongbloed LW, de Jong FH, van der Lely AJ, de Herder WW, Feelders RA. Differentiating between Cushing's disease and pseudo-Cushing's syndrome: comparison of four tests. European journal of endocrinology. 2014 Apr:170(4):477-86. doi: 10.1530/EJE-13-0702. Epub 2014 Mar 8
[PubMed PMID: 24394725]
[26]
Mezzullo M, Fanelli F, Fazzini A, Gambineri A, Vicennati V, Di Dalmazi G, Pelusi C, Mazza R, Pagotto U, Pasquali R. Validation of an LC-MS/MS salivary assay for glucocorticoid status assessment: Evaluation of the diurnal fluctuation of cortisol and cortisone and of their association within and between serum and saliva. The Journal of steroid biochemistry and molecular biology. 2016 Oct:163():103-12. doi: 10.1016/j.jsbmb.2016.04.012. Epub 2016 Apr 22
[PubMed PMID: 27108942]
Level 1 (high-level) evidence
[27]
Hariharan M, Naga S, VanNoord T, Kindt EK. Simultaneous assay of corticosterone and cortisol in plasma by reversed-phase liquid chromatography. Clinical chemistry. 1992 Mar:38(3):346-52
[PubMed PMID: 1547550]
[28]
Mezzullo M, Fazzini A, Gambineri A, Di Dalmazi G, Mazza R, Pelusi C, Vicennati V, Pasquali R, Pagotto U, Fanelli F. Parallel diurnal fluctuation of testosterone, androstenedione, dehydroepiandrosterone and 17OHprogesterone as assessed in serum and saliva: validation of a novel liquid chromatography-tandem mass spectrometry method for salivary steroid profiling. Clinical chemistry and laboratory medicine. 2017 Aug 28:55(9):1315-1323. doi: 10.1515/cclm-2016-0805. Epub
[PubMed PMID: 28076306]
Level 1 (high-level) evidence
[29]
Wood PJ, Barth JH, Freedman DB, Perry L, Sheridan B. Evidence for the low dose dexamethasone suppression test to screen for Cushing's syndrome--recommendations for a protocol for biochemistry laboratories. Annals of clinical biochemistry. 1997 May:34 ( Pt 3)():222-9
[PubMed PMID: 9158818]
[30]
Cronin C, Igoe D, Duffy MJ, Cunningham SK, McKenna TJ. The overnight dexamethasone test is a worthwhile screening procedure. Clinical endocrinology. 1990 Jul:33(1):27-33
[PubMed PMID: 2401096]
[31]
NUGENT CA, NICHOLS T, TYLER FH. DIAGNOSIS OF CUSHING'S SYNDROME; SINGLE DOSE DEXAMETHASONE SUPPRESSION TEST. Archives of internal medicine. 1965 Aug:116():172-6
[PubMed PMID: 14315650]
[32]
Bruno OD, Rossi MA, Contreras LN, Gómez RM, Galparsoro G, Cazado E, Kral M, Leber B, Arias D. Nocturnal high-dose dexamethasone suppression test in the aetiological diagnosis of Cushing's syndrome. Acta endocrinologica. 1985 Jun:109(2):158-62
[PubMed PMID: 2990131]
[33]
Biemond P, de Jong FH, Lamberts SW. Continuous dexamethasone infusion for seven hours in patients with the Cushing syndrome. A superior differential diagnostic test. Annals of internal medicine. 1990 May 15:112(10):738-42
[PubMed PMID: 2158760]
[34]
Jung C, Alford FP, Topliss DJ, Burgess JR, Long F, Gome JJ, Stockigt JR, Inder WJ. The 4-mg intravenous dexamethasone suppression test in the diagnosis of Cushing's syndrome. Clinical endocrinology. 2010 Jul:73(1):78-84. doi: 10.1111/j.1365-2265.2009.03756.x. Epub 2009 Dec 18
[PubMed PMID: 20039897]
[35]
Tran HA, Petrovsky N. Dexamethasone infusion testing in the diagnosis of Cushing's syndrome. Endocrine journal. 2005 Feb:52(1):103-9
[PubMed PMID: 15758565]
[36]
Yanovski JA, Cutler GB Jr, Chrousos GP, Nieman LK. Corticotropin-releasing hormone stimulation following low-dose dexamethasone administration. A new test to distinguish Cushing's syndrome from pseudo-Cushing's states. JAMA. 1993 May 5:269(17):2232-8
[PubMed PMID: 8386285]
[37]
Raff H, Carroll T. Cushing's syndrome: from physiological principles to diagnosis and clinical care. The Journal of physiology. 2015 Feb 1:593(3):493-506. doi: 10.1113/jphysiol.2014.282871. Epub 2015 Jan 5
[PubMed PMID: 25480800]
[38]
Bansal V, El Asmar N, Selman WR, Arafah BM. Pitfalls in the diagnosis and management of Cushing's syndrome. Neurosurgical focus. 2015 Feb:38(2):E4. doi: 10.3171/2014.11.FOCUS14704. Epub
[PubMed PMID: 25639322]
[39]
Pektas SD, Dogan G, Cinar N. Iatrogenic Cushing's Syndrome with Subsequent Adrenal Insufficiency in a Patient with Psoriasis Vulgaris Using Topical Steroids. Case reports in endocrinology. 2017:2017():8320254. doi: 10.1155/2017/8320254. Epub 2017 Nov 13
[PubMed PMID: 29259830]
Level 3 (low-level) evidence
[40]
Chabre O. The difficulties of pseudo-Cushing's syndrome (or "non-neoplastic hypercortisolism"). Annales d'endocrinologie. 2018 Jun:79(3):138-145. doi: 10.1016/j.ando.2018.04.017. Epub 2018 Apr 30
[PubMed PMID: 29716734]
[41]
Stephens MA, Wand G. Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol research : current reviews. 2012:34(4):468-83
[PubMed PMID: 23584113]
[42]
Bae YJ, Kratzsch J. Corticosteroid-binding globulin: modulating mechanisms of bioavailability of cortisol and its clinical implications. Best practice & research. Clinical endocrinology & metabolism. 2015 Oct:29(5):761-72. doi: 10.1016/j.beem.2015.09.001. Epub 2015 Sep 11
[PubMed PMID: 26522460]
[43]
Meikle AW. Dexamethasone suppression tests: usefulness of simultaneous measurement of plasma cortisol and dexamethasone. Clinical endocrinology. 1982 Apr:16(4):401-8
[PubMed PMID: 7094363]
[44]
Rush AJ, Schlesser MA, Giles DE, Crowley GT, Fairchild C, Altshuler KZ. The effect of dosage on the dexamethasone suppression test in normal controls. Psychiatry research. 1982 Dec:7(3):277-85
[PubMed PMID: 6962436]
[45]
Meinardi JR, Wolffenbuttel BH, Dullaart RP. Cyclic Cushing's syndrome: a clinical challenge. European journal of endocrinology. 2007 Sep:157(3):245-54
[PubMed PMID: 17766705]
[46]
Elamin MB, Murad MH, Mullan R, Erickson D, Harris K, Nadeem S, Ennis R, Erwin PJ, Montori VM. Accuracy of diagnostic tests for Cushing's syndrome: a systematic review and metaanalyses. The Journal of clinical endocrinology and metabolism. 2008 May:93(5):1553-62. doi: 10.1210/jc.2008-0139. Epub 2008 Mar 11
[PubMed PMID: 18334594]
Level 1 (high-level) evidence
[47]
Flack MR, Oldfield EH, Cutler GB Jr, Zweig MH, Malley JD, Chrousos GP, Loriaux DL, Nieman LK. Urine free cortisol in the high-dose dexamethasone suppression test for the differential diagnosis of the Cushing syndrome. Annals of internal medicine. 1992 Feb 1:116(3):211-7
[PubMed PMID: 1728204]
[48]
Aron DC, Raff H, Findling JW. Effectiveness versus efficacy: the limited value in clinical practice of high dose dexamethasone suppression testing in the differential diagnosis of adrenocorticotropin-dependent Cushing's syndrome. The Journal of clinical endocrinology and metabolism. 1997 Jun:82(6):1780-5
[PubMed PMID: 9177382]
[49]
Pecori Giraldi F, Pivonello R, Ambrogio AG, De Martino MC, De Martin M, Scacchi M, Colao A, Toja PM, Lombardi G, Cavagnini F. The dexamethasone-suppressed corticotropin-releasing hormone stimulation test and the desmopressin test to distinguish Cushing's syndrome from pseudo-Cushing's states. Clinical endocrinology. 2007 Feb:66(2):251-7
[PubMed PMID: 17223996]
[50]
Tirabassi G, Papa R, Faloia E, Boscaro M, Arnaldi G. Corticotrophin-releasing hormone and desmopressin tests in the differential diagnosis between Cushing's disease and pseudo-Cushing state: a comparative study. Clinical endocrinology. 2011 Nov:75(5):666-72. doi: 10.1111/j.1365-2265.2011.04096.x. Epub
[PubMed PMID: 21554373]
Level 2 (mid-level) evidence
[51]
Findling JW, Raff H. DIAGNOSIS OF ENDOCRINE DISEASE: Differentiation of pathologic/neoplastic hypercortisolism (Cushing's syndrome) from physiologic/non-neoplastic hypercortisolism (formerly known as pseudo-Cushing's syndrome). European journal of endocrinology. 2017 May:176(5):R205-R216. doi: 10.1530/EJE-16-0946. Epub 2017 Feb 8
[PubMed PMID: 28179447]
[52]
Pivonello R, De Martino MC, De Leo M, Lombardi G, Colao A. Cushing's Syndrome. Endocrinology and metabolism clinics of North America. 2008 Mar:37(1):135-49, ix. doi: 10.1016/j.ecl.2007.10.010. Epub
[PubMed PMID: 18226734]
[53]
Nieman LK, Ilias I. Evaluation and treatment of Cushing's syndrome. The American journal of medicine. 2005 Dec:118(12):1340-6
[PubMed PMID: 16378774]
[54]
Nieman LK. Diagnosis of Cushing's Syndrome in the Modern Era. Endocrinology and metabolism clinics of North America. 2018 Jun:47(2):259-273. doi: 10.1016/j.ecl.2018.02.001. Epub
[PubMed PMID: 29754631]
[55]
Poh DKH, Lim CY, Tan RZ, Markus C, Loh TP. Internal quality control: Moving average algorithms outperform Westgard rules. Clinical biochemistry. 2021 Dec:98():63-69. doi: 10.1016/j.clinbiochem.2021.09.007. Epub 2021 Sep 14
[PubMed PMID: 34534518]
Level 2 (mid-level) evidence
[56]
Kearney E. Internal quality control. Methods in molecular biology (Clifton, N.J.). 2013:1065():277-89. doi: 10.1007/978-1-62703-616-0_18. Epub
[PubMed PMID: 23996371]
Level 2 (mid-level) evidence
[57]
Bayat H. Selecting multi-rule quality control procedures based on patient risk. Clinical chemistry and laboratory medicine. 2017 Oct 26:55(11):1702-1708. doi: 10.1515/cclm-2016-1077. Epub
[PubMed PMID: 28236626]
Level 2 (mid-level) evidence
[58]
Cornish NE, Anderson NL, Arambula DG, Arduino MJ, Bryan A, Burton NC, Chen B, Dickson BA, Giri JG, Griffith NK, Pentella MA, Salerno RM, Sandhu P, Snyder JW, Tormey CA, Wagar EA, Weirich EG, Campbell S. Clinical Laboratory Biosafety Gaps: Lessons Learned from Past Outbreaks Reveal a Path to a Safer Future. Clinical microbiology reviews. 2021 Jun 16:34(3):e0012618. doi: 10.1128/CMR.00126-18. Epub 2021 Jun 9
[PubMed PMID: 34105993]
[59]
Munson E, Bowles EJ, Dern R, Beck E, Podzorski RP, Bateman AC, Block TK, Kropp JL, Radke T, Siebers K, Simmons B, Smith MA, Spray-Larson F, Warshauer DM. Laboratory Focus on Improving the Culture of Biosafety: Statewide Risk Assessment of Clinical Laboratories That Process Specimens for Microbiologic Analysis. Journal of clinical microbiology. 2018 Jan:56(1):. doi: 10.1128/JCM.01569-17. Epub 2017 Dec 26
[PubMed PMID: 29118166]
[60]
Ionescu G, Neguţ M, Combiescu AA. [Biosafety and biosecurity in the medical laboratory. Update and trends]. Bacteriologia, virusologia, parazitologia, epidemiologia (Bucharest, Romania : 1990). 2007 Jul-Dec:52(3-4):91-9
[PubMed PMID: 19326721]