Continuing Education Activity
Approximately 1 million hip and knee arthroplasties are performed annually in the United States, and this number is expected to quadruple over the next 10 to 20 years as the population ages. The incidence of periprosthetic joint infection (PJI) is 1% to 2%, and PJI is now the primary indication for revision arthroplasty. Unfortunately, PJI is not a straightforward diagnosis; there is no uniformly accepted definition of PJI, and PJI can occur anywhere from 4 weeks to 2 years following primary arthroplasty. PJI has a profound physical, social, and emotional impact on affected patients and significantly increases healthcare expenditures, patient morbidity, and overall mortality.
This activity reviews the epidemiology, etiology, clinical symptomatology, evaluation, and management of PJI and highlights the critical role of the interprofessional healthcare team in caring for patients with this potentially life-altering diagnosis.
Objectives:
Differentiate among early, delayed, and late periprosthetic joint infections based on clinical presentation.
Apply best practices when evaluating patients with a possible periprosthetic joint infection.
Analyze the objective clinical findings of patients with periprosthetic joint infection using validated diagnostic criteria to determine the optimum clinical management.
Develop and implement interprofessional team strategies to effectively care for patients with periprosthetic joint infections.
Introduction
Periprosthetic joint infection (PJI) is a unique clinical entity, markedly different from infections involving native bones or joints.[1] PJI is characterized by a complex interplay between microbes, predominantly bacteria but occasionally fungi, and the host immune response. Only a minimal microbial burden is required to initiate a PJI; etiologic organisms can adhere to the surfaces of arthroplasty components and form biofilms.[1][2][3] Biofilms notoriously exhibit a marked resistance to a wide array of antimicrobial agents and are adept at avoiding innate immune defenses.[2][3] The offending microorganisms in PJI typically originate from the skin microbiome and may be introduced during the perioperative phase of the implantation procedure.[3] Alternatively, these pathogens can seed the implant postoperatively via hematogenous dissemination or direct inoculation from adjacent infected tissues.[4][5]
There is no uniformly accepted definition for PJI.[2][4][5] The clinical presentation of PJI varies; the classical features of infection, such as fever, leukocytosis, and signs of sepsis, are often absent.[4] A prosthesis is a foreign body, and its mere presence is a risk factor for infection.[6] The microbial load needed to produce an infection in a prosthetic joint is much less than that for a native joint.[7]
The strongest indicating factor of a PJI is a joint aspirate or surgically obtained periprosthetic material that yields a microorganism when cultured.[2] Other findings indicating a PJI include a sinus tract communicating with the joint space, wound dehiscence, gross purulence, prosthetic loosening, synovial fluid leukocytosis with neutrophilia, and elevated serum inflammatory markers.[3] However, failure to identify an etiologic pathogen does exclude the presence of PJI.[7]
Periprosthetic joint infections are a significant clinical problem. PJIs significantly increase morbidity and mortality and have surpassed polyethylene wear as the primary indication for revision arthroplasty.[8][9] This activity will review the epidemiology, etiology, pathophysiology, evaluation, and management of PJI and highlight the role of the interprofessional healthcare team in reducing the risk of PJI development and caring for patients who develop this problematic complication of joint replacement.
Etiology
The etiologic agents of PJI comprise diverse bacteria and fungi.[10] A single tertiary referral center study revealed that coagulase-negative staphylococci, particularly Staphylococcus epidermidis, are the most frequently isolated pathogens from PJIs. Staphylococcus aureus and various species of Streptococcus, Enterococcus, Cutibacterium, and Enterobacterales are also commonly encountered in PJIs.[8] Approximately 70% of the PJIs in this cohort were monomicrobial, and 25% were polymicrobial.[8] Other studies have identified C acnes as the causative agent in approximately 44% of shoulder PJIs.[11]
Periprosthetic joint infections are classified according to when they occur in relation to the insertion of the prosthetic joint. The presumptive etiologic agents of each classification differ. Common classification systems define PJIs as early, delayed, or late infections.[4][5]
Early PJIs occur within the initial 4 weeks following the primary arthroplasty. Early PJIs are typically caused by highly virulent organisms such as S aureus, aerobic gram-negative bacilli, beta-hemolytic streptococci, and Enterococcus spp.
Delayed PJIs occur between 3 and 12 months following primary arthroplasty. These infections are typically caused by organisms of lesser virulence, including coagulase-negative staphylococci, C acnes, and enterococci. S aureus may cause a delayed PJI, but at a decreased frequency compared to early PJI.
Late PJIs occur 1 to 2 years after primary arthroplasty and are typically hematogenous in nature. Common etiologic agents of late PJIs include S aureus,coagulase-negative staphylococci, viridans streptococci, enterococci, and occasionally gram-negative bacilli.
The proportion of culture-negative PJIs varies significantly across studies, a discrepancy attributable to differences in diagnostic methodologies, prior antibiotic exposure, and the criteria employed to define culture positivity.[6][7] The rate of reported culture-negative PJIs may reach 45%.[11]
Epidemiology
Approximately 1 million hip and knee arthroplasties are performed annually in the United States; this number is expected to quadruple over the next 10 to 20 years.[10][12] There has been a simultaneous parallel escalation in the incidence of hip and knee PJIs, which is similarly projected to continue.[9][13] The incidence of PJI following hip and knee arthroplasty is estimated at 1% to 2%. However, there is significant variability in reported incidence rates across different studies, attributable to diverse patient populations, varying definitions of PJI, and differing durations of postoperative follow-up.[13][14]
While the risk of developing PJI is most pronounced in the early postoperative period, PJI is a persistent risk extending throughout the lifespan of the prosthesis.[15] A significant number of PJIs become clinically apparent after the first postoperative year. For example, in population-based studies, the incidence of knee PJI was observed to escalate from 0.8% at 1 year to 2.0% at the 15-year mark.[16] Similarly, a Canadian study reported the incidence of hip PJI to be 0.5% at 1 year and increasing to 1.4% at 15 years.[17] PJI has surpassed polyethylene wear and is now the primary indication for revision arthroplasty.[8][9]
Risk Factors for Developing Periprosthetic Joint Infection
The risk factors for developing a PJI include preoperative, modifiable, nonmodifiable host, surgery-related, and postoperative factors.
Preoperative factors that increase the risk of developing a PJI include a history of prior surgery at the arthroplasty site, current bacteremia or sepsis, and a previous or active infection at the current surgical site. Patients with a history of multiple arthroplasties and a prior PJI may have up to a 20% risk of another synchronous or metachronous PJI. Additionally, the inadequate use of evidence-based PJI preventative measures significantly increases the risk of developing a PJI.
Modifiable risk factors for PJI include current tobacco use, excessive alcohol use, intravenous drug use, poor oral hygiene, malnutrition, poor preoperative glycemic control, and obesity. Patients with a BMI greater than 40 are at significantly increased risk of PJI.[18]
Data indicates the possibility of nonmodifiable genetic factors predisposing to developing a PJI, although the specific genes have not been identified. Patients with a PJI who also had a first- or second-degree relative with a history of PJI were at increased risk of PJI development, even after adjusting for socioeconomic status.
Intra-articular injections of glucocorticoids, hyaluronic acid, or anesthetics in the 3 months preceding arthroplasty increase the risk of PJI development. Similarly, medical comorbidities resulting in immunosuppression also increase the risk of PJI development. Such comorbidities may include but are not limited to poorly controlled diabetes mellitus, acute liver injury, chronic kidney disease, infection with the human immunodeficiency virus (HIV), inflammatory arthropathies, and the use of immunosuppressant or immune-modifying medications such as systemic corticosteroids or disease-modifying antirheumatic agents.
For reasons that are not well understood, patients with Medicaid as their primary insurer exhibit an elevated PJI risk, even after adjusting for educational attainment and household income.
Surgery-related factors that increase the risk of PJI include an extended surgical time of more than 90 minutes and increasing complexity of the procedure itself. Patients with a significant degree of varus, valgus, or flexion deformity undergoing total knee arthroplasty are at significantly increased risk of PJI, as are patients with dysplasia undergoing total hip arthroplasty.[18] Patients who have undergone primary arthroplasty and develop a hematoma, seroma, or wound dehiscence in the postoperative period are also at increased risk of PJI development.
Proactive Measures to Reduce Periprosthetic Joint Infections
In addition to addressing modifiable risk factors, other proactive measures may be taken to reduce the risk of PJI development. Patients undergoing arthroplasty in low-volume facilities by surgeons with limited experience are at increased risk of developing a PJI.[8] Preoperative screening for and decolonization of S aureus is advised; meta-analyses demonstrate an increased risk of PJI without decolonization.[19] Patients should be advised to cleanse their skin preoperatively using chlorhexidine cloths or soap and water.[20]
Surgical-site preparation protocols based on best practices and minimizing operating room traffic reduce the risk of PJI.[18]
The administration of cefazolin within 1 hour of the surgical incision and before tourniquet inflation has also been shown to reduce the incidence of PJI. Most patients with a self-reported penicillin allergy can be given cefazolin without adverse effects unless the allergic reaction is characterized by anaphylaxis or Stevens-Johnson syndrome.[21] These patients may benefit from a formal allergy evaluation preoperatively. For MRSA-colonized patients, a combination of vancomycin and cefazolin is sometimes recommended. However, alternative antibiotic regimens, such as vancomycin and clindamycin, have shown a higher association with PJI across various joints. Ongoing trials are evaluating the efficacy of different prophylactic regimens, including extended oral antibiotic prophylaxis in high-risk populations.[21]
Avoiding aggressive anticoagulation for deep venous thrombosis (DVT) prophylaxis is recommended; aspirin is preferred. While the intraoperative use of vancomycin powder remains controversial, dilute povidone-iodine lavage appears beneficial in reducing the risk of PJI.
Pathophysiology
The formation of a biofilm is a critical pathophysiological process in the development of a PJI. Many bacteria, including coagulase-negative staphylococci, have surface adhesins and can produce extracellular polysaccharide biofilms, promoting adherence to foreign bodies such as catheters and prosthetic devices, including joint replacement components. Biofilms are a barrier to antimicrobial agents, requiring a much higher antibiotic concentration to achieve bactericidal activity than planktonic bacteria.[22] Biofilms typically mature over 4 weeks, and their presence significantly impacts the selection of therapeutic regimens.[23]
Additionally, certain strains of S aureus can form slow-growing, small-colony variants, leading to recurrent and challenging infections.[23]
The etiologic agents of PJIs may reach the prosthetic joint via hematogenous or localized dissemination; hematogenous dissemination is the most common seeding method.[9] Frequent sources of hematogenous dissemination include:
- Skin and soft tissue infections (S aureus)
- Lower respiratory tract infections (Streptococcus pneumoniae)
- Urinary tract infections (Escherichia coli, Enterobacterales spp, including Klebsiella).
- Gastrointestinal infections (Bacteroides, Salmonella, Streptococcus gallolyticus).
- Recent dental procedures (viridans streptococci)
- Infected intravascular devices (S epidermidis)
Localized dissemination most commonly stems from a nearby septic focus, such as osteomyelitis or surrounding soft tissue infection, or in circumstances of direct trauma, as seen with open periprosthetic fractures.[9][15]
History and Physical
The predominant clinical manifestation of PJI is pain within the affected joint.[2][4] Local inflammatory signs, including erythema, joint swelling, and warmth, may be evident in some patients; systemic manifestations of infection, such as fever, are frequently absent.[24] Chronic infections may present subtly, with pain as the sole symptom, or conspicuously when associated with prosthetic loosening or a draining sinus tract. While a draining sinus is pathognomonic for PJI, its absence does not preclude the diagnosis.[25] The clinical differentiation between PJI and noninfectious etiologies of arthroplasty failure is a nuanced yet critical distinction that significantly impacts surgical and medical therapeutic strategies.[23][25]
As aforementioned, PJIs may present as early, delayed, or late infections.[24] Early infections are typically caused by virulent organisms acquired in the perioperative period and present acutely with erythema, edema, incisional induration, and wound drainage. The etiologic agents of delayed infections may also be acquired perioperatively; these organisms are typically less virulent. Late PJIs are usually hematogenous and often without fever, draining wounds, or local signs of infection.[25]
Overall, the symptoms of PJI are nonspecific. Most patients present with joint swelling or persistent and progressively worsening pain. Reports of joint instability and difficulty ambulating in PJIs affecting the lower extremities are not unusual.[26]
Evaluation
Accurately diagnosing a PJI requires combining subjective symptoms with objective physical examination findings and laboratory evaluation results such as synovial fluid cell counts, serum inflammatory markers, and culture results.[4][24][25] The defining criteria for PJI have evolved from the 2012 guidelines by the Infectious Diseases Society of America and the 2011 Musculoskeletal Infection Society to criteria proposed by international consensus groups and societies between 2018 and 2021.[27][28] The diagnostic threshold for a PJI is much lower than for septic arthritis of a native joint.[8] For example, synovial fluid leukocyte counts in cases of septic arthritis are typically tens of thousands, but a synovial fluid leukocyte count greater than 4200 cells/μL supports the diagnosis of a hip PJI, and greater than 1700 cells/μL supports the diagnosis of a knee PJI.[28] Neutrophil predominance of >80% is a typical feature for both hip and knee PJIs.[28]
Blood and Synovial Fluid Cultures
Blood and synovial fluid cultures are critical tools when diagnosing PJIs.[29] Synovial fluid should be cultured using multiple sets of bottles in aerobic and anaerobic environments; mycobacterial and fungal cultures may be performed if clinically indicated.[8] Anaerobic cultures should be maintained for 14 days.[30] The synovial fluid should also be analyzed for total leukocyte count and neutrophil percentage. Assays for alpha-defensin, C-reactive protein, leukocyte esterase, and calprotectin can be conducted on synovial fluid but often provide overlapping diagnostic information.[31] If the clinical relevance of an organism isolated from synovial fluid is ambiguous, a second joint aspiration may be warranted.
Blood cultures reveal an organism in approximately 25% of PJIs, most of which are early infections. Unfortunately, organisms identified through blood culture do not always match those identified through synovial fluid culture.[32] Proteomic and genomic diagnostics, such as next-generation sequencing, have shown promise in identifying PJI etiologic agents but have not been widely adopted in clinical practice.[30]
Imaging Techniques
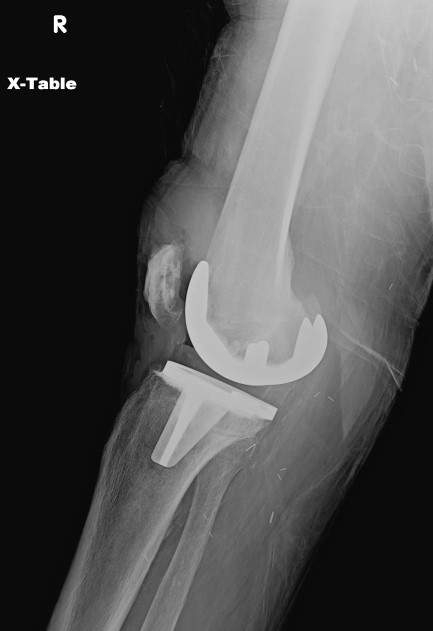
Plain radiography offers limited sensitivity and specificity for PJI; PJi and aseptic loosening have similar radiographic findings (see Image. Radiograph of Periprosthetic Joint Infection). Plain radiography of PJI may reveal joint effusion or malalignment, bone-cement-metal interface lucencies, periosteal reactions, or patchy osteolysis.[26] Periprosthetic bone resorption or transcortical sinus tracts may be seen in cases of PJI.[33][34]
The evaluation of PJI using advanced imaging techniques such as Fludeoxyglucose F 18 (FDG)-positron emission tomography (PET) or technetium Tc 99m scanning offers limited diagnostic utility. However, these imaging studies may reveal bony erosion, abscess or sinus tract formation, or prosthesis loosening.[31]
Intraoperative Assessment
Even if the preoperative synovial fluid culture reveals an etiologic agent consistent with PJI, tissue cultures are crucial to the investigation as many PJIs are polymicrobial. Given the limited sensitivity of individual cultures and the need to differentiate between genuine pathogens and contaminants, multiple intraoperative samples are needed.[35] Confirming the microbiological diagnosis of PJI requires detecting an identical microorganism across 2 or more samples. Abstaining from antibiotics for at least 2 weeks before tissue culture can enhance diagnostic yield.[36] If implant components are removed, sonication techniques can be employed to culture their surfaces and identify biofilms.[35]
Diagnostic Criteria for Periprosthetic Joint Infection
The 2018 Musculoskeletal Infection Society (MSIS) definition of hip and knee PJI is evidence-based and validated criteria.[28] The criteria are a scoring-based definition consisting of major and minor criteria. The criteria should be used cautiously for adverse local tissue reaction, crystal deposition disease, or slow-growing organisms. The diagnosis of PJi can be made if one major criterion is met. (see Table 1. 2018 MSIS Major Criteria for the Diagnosis of PJI)
Table 1. 2018 MSIS Major Criteria for the Diagnosis of PJI
| Major Criteria (at least one of the following) |
Decision |
| Two positive cultures of the phenotypically identical organism |
Infected |
| Sinus tract with evidence of communication to the joint of visualization of the prosthesis |
Infected |
The diagnosis of PJI may also be made in circumstances where preoperative findings score ≥6. A score between 2 and 5 is inconclusive and means the patient possibly has a PJI but requires additional findings to confirm the diagnosis. A score of 0 or 1 is negative for PJI. (see Table 2. 2018 MSIS Minor Criteria for the Diagnosis of PJI)
Table 2. 2018 MSIS Minor Criteria for the Diagnosis of PJI
| Minor Criteria (based on preoperative diagnosis) |
Score (points) |
| Elevated serum C-reactive protein or D-dimer |
2 |
| Elevated serum erythrocyte sedimentation rate (ESR) |
1 |
| Elevated synovial fluid total leukocyte count or leukocyte esterase |
3 |
| Positive synovial fluid alpha-defensin |
3 |
| Elevated synovial fluid neutrophil percentage |
2 |
| Elevated synovial fluid C-reactive protein |
1 |
| Decision (total score points) |
Result |
| Score ≥6 |
Infected |
| Score 4 or 5 |
Inconclusive |
| Score ≤3 |
Not infected |
If major criteria are not met, preoperative diagnostic testing yields inconclusive results, or the synovial fluid aspiration does not produce adequate fluid for testing, intraoperative diagnosis will be required. (see Table 3. 2018 MSIS Intraoperative Criteria for the Diagnosis of PJI)
Table 3. 2018 MSIS Intraoperative Criteria for the Diagnosis of PJI)
| Intraoperative Diagnosis |
Score (points) |
| Preoperative Score |
- |
| Positive histology |
3 |
| Positive purulence |
3 |
| Single positive culture |
2 |
| Decision (total intraoperative diagnosis score points) |
Decision |
| Score ≥6 |
Infected |
| Score 4 or 5 |
Inconclusive |
| Score ≤3 |
Not infected |
If the intraoperative diagnostic score remains inconclusive, molecular diagnostic testing such as next-generation sequencing is recommended.[28]
Treatment / Management
Managing a PJI requires a multidisciplinary team; treatment frequently combines surgical management and prolonged intravenous (IV) antibiotic courses for 6 or more weeks.[37] Treatment of PJI may aim to be curative, with eradication of infection, return of joint function, and alleviation of symptoms, or be palliative only, comprising suppressive antibiotics, joint fusion, and symptom control.
Antibiotic Therapy
Antibiotic therapy is almost always necessary but should be delayed for culture after blood and synovial fluid are obtained.[30] The rare exception is in situations of sepsis or overwhelming infection. Empirical antibiotic therapy should be tailored according to the timing of infection, be it early, delayed, or late, but should provide adequate coverage against S aureus, including methicillin-resistant S aureus (MRSA), coagulase-negative staphylococci, and aerobic gram-negative bacilli.[32][29] Antibiotics with excellent activity against biofilms, such as rifampin and fluoroquinolones, are often included in empirical treatment. Other possible oral antibiotic choices for prosthetic joint infection include minocycline, linezolid, and trimethoprim-sulfamethoxazole.[29]
Surgical Management Options
Debridement and implant retention (DAIR): With this approach, the metallic implants are left in situ, but the joint cavity is debrided with the exchange of the modular polyethylene liner components.[38] This is the typical surgical approach for early PJI, provided the implant is stable, symptom duration is less than 3 weeks, no sinus tracts are present, and the isolated pathogen is susceptible to active biofilm antibiotics. Another indication for DAIR is an acute hematogenous infection with less than 72 hours of symptoms. DAIR followed by an extended course of IV antibiotics.
One-stage exchange: This is the surgical approach commonly employed in Europe; it is not the standard of care in the United States.[39] A one-stage exchange removes the entire infected prosthesis and replaces a new set of definitive implants within the same procedure. Patient selection is critical in a one-stage exchange and is indicated only in the immunocompetent patient with minimal medical comorbidities and healthy soft tissues. The etiologic organism has to be of low virulence, and antibiotic sensitivities must be known preoperatively. Compared to a two-stage exchange, the one-stage exchange offers benefits such as reduced costs, shorter hospital stays, and improved patient mobilization but carries an increased risk of residual infection. Current clinical studies are evaluating the differences in outcomes between the two approaches.
Two-stage exchange: This approach is the standard of care in the United States. The 2-stage exchange offers the best chance of cure, especially for delayed and late PJIs.[39] The most simplified version of the procedure involves complete removal of the infected prosthesis, placement of an antibiotic-impregnated joint spacer, IV antibiotic therapy for 2 to 8 weeks, and subsequent placement of a new prosthesis. Patients must be candidates for multiple procedures and have adequate bone stock. The decision on reimplantation requires a supporting clinical examination, normal laboratory studies, and negative cultures 2 weeks after completion of IV antibiotics.
Salvage Options
Salvage options are employed when patients are poor surgical candidates for a 2-stage exchange, or the aforementioned surgical options have failed. Chronic suppressive antibiotic therapy may be a suitable approach for patients unsuitable for surgery, especially those who are bedridden, debilitated, or have multiple severe comorbidities.[40] However, this treatment often falls short of completely eradicating the infection, leading to a need for lifelong antibiotic administration.[41]
In situations where postsurgical joint functionality is anticipated to be suboptimal, or if the infection remains despite surgical interventions, resection arthroplasty with the removal of the prosthesis without replacement may be considered. Resection arthroplasty is usually reserved for elderly, nonambulatory patients with high operative risks or patients for whom a prosthesis exchange offers no functional advantage.[40][41] However, resection arthroplasty is a viable option for patients with inadequate bone stock, compromised soft tissues, recurrent infections, or a history of multiple unsuccessful revision surgeries.
In extreme cases of knee PJI, when all conservative and surgical measures fail, or there is significant vascular compromise or bone or soft tissue loss, an above-knee amputation may be necessary.[42]
Differential Diagnosis
The primary challenge in diagnosing PJI is distinguishing it from aseptic complications. Aseptic complications manifest with symptoms such as pain, joint swelling, skin redness, or limited joint mobility, closely resembling PJI.[41] While complications such as increased wear, osteolysis, or cement debonding are evidence of aseptic malfunction, concomitant PJI must be excluded before proceeding with any corrective surgery, given that approximately 12% of aseptic cases have an underlying PJI.[43]
Other disease processes that must be considered in the differential diagnosis of PJI include hemarthrosis and crystalline deposition disease; synovial fluid analysis will demonstrate sanguineous fluid with a high percentage of red blood cells in hemarthroses or crystals in crystalline deposition diseases. Referred pain from the spine or hip may masquerade as PJI, as will systemic neuropathic, vascular, or inflammatory conditions. Additionally, metal-on-metal complications such as aseptic lymphocyte-dominant vasculitis-associated lesions and adverse tissue reactions may present with symptoms similar to PJI; leukocyte migration or lymphocyte transformation testing may be helpful.
Prognosis
Inappropriately treated or missed PJIs can result in the persistence of infection with deleterious consequences, including disability and impaired quality of life.[42] A significant financial burden, extensive time commitment, and substantial utilization of healthcare resources characterize the management of PJI. Compared to those with noninfected hip replacements, PJI patients experience diminished life quality, impaired joint functionality, and a higher likelihood of requiring mobility aids and assisted living.[42]
Complications
PJI is linked with prolonged hospital stays, suboptimal treatment outcomes, elevated disability rates, diminished life quality, and increased mortality when contrasted with noninfected joint replacements.[42]
Patients with PJI have significantly more prolonged hospitalizations than those undergoing primary arthroplasty: 7.6 d vs. 3.3 d for hip replacements and 5.3 d vs. 3.0 d for knee replacements.[44] There are no large randomized-controlled clinical trials on PJIs; outcomes are based on small retrospective case series and case reports.
The success of the surgical interventional procedures for PJI depends on the severity and duration of the infection, time of treatment, and patient comorbidities. The average success rate for infection eradication following one-stage and two-stage knee replacement revisions is 87% and 83%, respectively.[32][45] However, there is significant selection bias; many patients do not undergo the second stage of a two-stage revision, and the completion rate is below 50%. Furthermore, success rates for different PJI stages varied: 74% for early, 49% for delayed, and 44% for chronic PJI.[46]
For two-stage revisions, the one-year post-explantation mortality rates are 13% for hip replacements and 9% for knee replacements. The 5-year mortality rate after hip-related PJI is 21%, a fourfold increase compared to age-adjusted rates; 10-year mortality rates are 45%.[46][47]
Deterrence and Patient Education
PJI has a profound physical, social, and emotional impact on patients.[44] These impacts are attributed to recurrent hospital admissions, expensive repeat surgeries, extended hospitalizations, augmented outpatient service usage, and extended antibiotic regimens. The repercussions of PJI include reduced physical capability, bed confinement, extended antibiotic courses, loss of independence, and heightened fears of disease escalation or mortality.[42] These factors culminate in psychological distress, feelings of isolation, and elevated levels of depression and anxiety, comparable to cancer patients. The extended recovery period can induce significant psychological strain even in successful cases. For medical professionals, especially surgeons, PJI can cause feelings of guilt and cognitive strain, potentially leading to professional burnout.[48]
Hospital expenditure per PJI episode is estimated at approximately $89,000 for hip infections and $116,000 for knee infections.[49] By 2030, it is projected that hospital expenses for hip and knee PJI in the United States will escalate to an estimated annual total of $1.85 billion.[49] Current Medicare reimbursement rates for PJI management are widely regarded as insufficient and necessitate revision to align with these elevated costs, ensuring sustained patient access to high-quality care. This is particularly pertinent given that existing reimbursement gaps may disproportionately affect underserved populations.[50]
Enhancing Healthcare Team Outcomes
The last 20 years have significantly improved comprehension of PJI as a distinct medical condition. For patients with PJI, personalized treatment at specialized centers, much like the model of care provided in cancer centers, is recommended. The diversity of surgical scenarios, myriad etiologic microorganisms, and rising antibiotic resistance necessitate specialized and personalized care. [51]
The management of a PJI is best done with an interprofessional team consisting of an orthopedic surgeon experienced in arthroplasty and its complications, an infectious disease expert, a dietician, a rehabilitation specialist, nurses, and pharmacists.[40] Patients with PJI are often confined to bed for long periods, and a physical therapy consultant is recommended for joint movement and muscle training. A dietary consult may help prevent muscle wasting, and the nurse should ensure that the patient has prophylaxis against DVT and pressure ulcers. Pharmacists help tailor antibiotic regimens to isolated susceptibilities and patient comorbidities.
After discharge, most patients need physical therapy for months to regain joint motion and muscle strength. Given the profound social and psychological impacts of PJI, integrating a psychologist into the interprofessional care team may be warranted.[52][53][54]