Continuing Education Activity
Managing severe acute kidney injury demands a nuanced understanding of renal replacement therapies. Among the options, including intermittent hemodialysis and prolonged intermittent renal replacement therapy, continuous renal replacement therapy (CRRT) emerges as a preferred modality in various clinical scenarios. A multinational study revealed that CRRT is utilized in 75.2% of intensive care unit visits. CRRT, involving continuous solute removal and fluid balance techniques, employs 3 distinct modalities: continuous venovenous hemofiltration, continuous venovenous hemodialysis, and continuous venovenous hemodiafiltration. CRRT's indications span volume overload, electrolyte disturbances, and uremia complications. This intervention excels in managing hemodynamically unstable patients, allowing controlled fluid management and mitigating risks associated with rapid solute changes.
Collaboration in CRRT implementation involves critical nephrology specialists, a proficient CRRT nursing team, and interdisciplinary cooperation. Recognizing associated risks, including intravascular line complications and therapy-related electrolyte disturbances, underscores the importance of a comprehensive approach. CRRT demands an interprofessional healthcare team, incorporating critical care, nephrology, neurology, nursing, pharmacy, and nutrition, each playing a crucial role in patient care, safety, and therapeutic success.
This activity for healthcare professionals is designed to enhance learners' proficiency in identifying patients with indications for CRRT and administering the intervention properly. Participants gain a comprehensive understanding of CRRT's underlying principles, optimal dosing, and potential complications. Learners acquire the skills and competencies necessary to deliver high-quality CRRT, equipping them to collaborate effectively within an interprofessional team and optimize outcomes for patients with acute or chronic kidney injury.
Objectives:
Identify patients with continuous renal replacement therapy indications based on clinical evaluation and diagnostic test results.
Determine the most suitable continuous renal replacement therapy modality for a patient with kidney failure.
Evaluate continuous renal replacement therapy effectiveness to allow for therapy optimization.
Implement effective interprofessional communication practices to optimize outcomes for patients on continuous renal replacement therapy.
Introduction
Various renal replacement therapies (RRTs) are available for managing severe acute kidney injury (AKI), including intermittent hemodialysis (IHD), continuous renal replacement therapy (CRRT), and prolonged intermittent RRT. Decisions about technique are dictated by the dialysis indication, clinician preference, outcome data, and, most importantly, hemodynamic status.[1] A 2015 multinational cross-sectional epidemiological study of patients with AKI in intensive care units (ICUs) revealed that CRRT was the preferred treatment modality in 75.2% of sessions, compared to intermittent dialysis in 24.1% of sessions and peritoneal dialysis in 0.7% of sessions.[2]
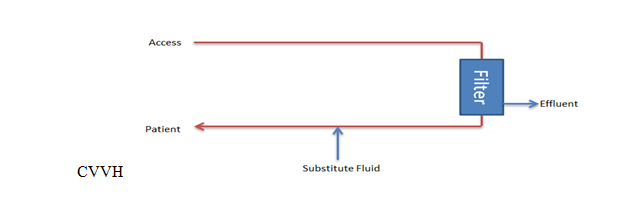
CRRT comprises techniques that manage solute removal and fluid balance over 24 hours. CRRT filters blood through a semipermeable membrane using various solute transport mechanisms. The specific mechanism defines each CRRT type. The 3 CRRT techniques are continuous venovenous hemofiltration (CVVH), continuous venovenous hemodialysis (CVVHD), and continuous venovenous hemodiafiltration (CVVHDF).
Anatomy and Physiology
Vascular Access for CRRT
CRRT vascular access employs a dual-lumen catheter placed in either the internal jugular or femoral vein. A nontunneled dual-lumen catheter may be placed if CRRT is only needed in the short term. However, prolonged CRRT use requires either a cuffed or noncuffed tunneled dual-lumen catheter. These vascular access types allow a blood flow rate of 200 to 250 mL/min. Previously established access, such as arteriovenous fistulae or grafts, should not be utilized for CRRT due to possible graft dislodgement or injury.
The right internal jugular vein is the most preferred option for direct access to the superior vena cava as it ensures optimal blood flow.[3] The left internal jugular or femoral veins may be considered in patients with obesity, though these vessels are less favored due to potential blood flow impairment with positional changes. Femoral vein use was traditionally reserved for patients with anatomical internal jugular vein obstruction, eg, stenosis or thrombosis. However, the femoral veins are also currently widely used for CRRT vascular access in patients without obesity. Using the subclavian vein for CRRT vascular access is discouraged because it poses a risk of stenosis, which can lead to difficulties using downstream vessels for future arteriovenous fistulae or grafts.
Patient height and the insertion site dictate the necessary length of the vascular catheter. Right-sided internal jugular catheters typically range from 12 to 15 cm, while left-sided internal jugular catheters are 20 to 24 cm long. Femoral catheters are at least 24 cm in length. Nontunneled catheter placement is typically performed at the bedside under aseptic conditions using ultrasound guidance per the Kidney Disease Improving Global Outcomes (KDIGO) guidelines. Tunneled catheters are placed in the angiography suite with venous cannulation using ultrasound guidance and catheter tip positioning via fluoroscopic guidance. Confirming catheter tip positioning in the superior vena cava via chest radiography is typically required before using nontunneled catheters. The ideal catheter tip location is close to or within the inferior vena cava if a femoral vein is used for vascular access. A higher chance of restricted blood flow, circuit flow interruptions, and filter clotting is anticipated if the catheter is malpositioned.[4]
Principles of CRRT
CRRT aims to mimic the kidney's countercurrent mechanism to achieve effective solute and plasma water transport. Solute transport is achieved either by diffusion, convection, or both. Slow blood flow mechanisms ensure plasma water and effluent equilibration. Diffusion is the primary solute transport method for substances with a molecular weight of less than 1000 Da. Convective transport permits the movement of intermediate molecular weight molecules via high hydrostatic pressure. Convective transport filters molecules with a maximum weight of approximately 15,000 Da.
In contrast to IHD, CRRT dialysate flow rates are far lower than blood flow rates, with an average difference of 15 to 30 mL/min. Small solutes in the plasma water can equilibrate with the effluent at this flow rate, effectively making solute clearance equal to the effluent fluid rate (Qef) or the final output postfiltration. This value comprises the net ultrafiltration (Qnet) plus the replacement fluid rate (QRF) in CVVH. The dialysate flow rate is also considered in CVVHD and CVVHDF.
Effluent
The primary goal of CVVH is to regulate the total ultrafiltrate, which comprises both the replacement and net fluid removed from the patient's bloodstream (see Image. Continuous Venovenous Hemofiltration Circuit). Replacement fluid is administered to maintain proper fluid balance, while net fluid removal represents the volume of fluid extracted during filtration to alleviate fluid overload or remove waste products.
CVVHD involves the utilization of spent dialysate, the fluid that has passed through the dialyzer, along with net fluid removal. This process aids in removing waste substances and excess fluids from the blood (see Image. Continuous Venovenous Hemodialysis Circuit). Conversely, CVVHDF integrates both spent dialysate and total ultrafiltrate. Spent dialysate aids solute removal, while total ultrafiltrate accounts for the combined volume of replacement fluid and net fluid removed, ensuring comprehensive management of fluid and solute balance.
General clearance
The general clearance (K) equation quantitatively measures how efficiently CRRT removes solutes from the blood based on the effluent flow rate and solute concentration gradient between the effluent and the blood. The equation is as follows:
K= QE x CE/CB, where CE/CB is the sieving coefficient, S
where
- "K" represents the clearance or the rate at which solutes are removed from the blood by the CRRT process. K is measured in milliliters per minute and indicates how efficiently the therapy removes solutes.
- "QE" refers to the effluent flow rate, which is the rate at which the dialyzed or filtrate fluid is removed from the patient's blood during CRRT. This quantity is typically measured in milliliters per minute and represents the volume of fluid processed by the CRRT machine.
- "CE" represents the concentration of solutes in the effluent fluid, which is the fluid that has been filtered or dialyzed and removed from the patient's bloodstream. CE is measured in concentration units (eg, milligrams per deciliter).
- "CB" represents the concentration of solutes remaining in the patient's bloodstream after passing through the CRRT circuit. CB is also measured in concentration units (eg, milligrams per deciliter).
A solute with an S of 1 can pass freely through a filter. If S is 0, the solute cannot pass through the filter.[5][6] This factor and the difference in hydrostatic pressure between the dialysate and blood compartments determines the amount of solute that convection will remove. The amount of solute that diffuses through the membrane depends on its concentration gradient, the solute's molecular size, and the membrane's thickness, surface area, and pore size.
Indications
RRT indications in the acute setting often include the same dialysis indications in acute renal failure, such as volume overload, acidosis, electrolyte disturbances like hyperkalemia, and uremia complications. The patient's hemodynamics drive the modality choice. CRRT is employed when signs of hemodynamic instability become evident due to reduced blood flow. This modality allows more controlled fluid management and greater net fluid removal than IHD.[7][8][9]
Additionally, CRRT can achieve better solute control in patients with high catabolic rates or have rapid cell breakdown, as in individuals with tumor lysis syndrome or rhabdomyolysis. CRRT gradually eliminates osmotically active substances, mitigating intracranial pressure increases and facilitating controlled mean arterial pressure adjustments. Consequently, CRRT emerges as the preferred treatment modality for patients experiencing cerebral edema.[10][11]
IHD is the modality of choice for life-threatening hyperkalemia and acute intoxications because of its ability to remove toxins and excess potassium through higher blood flow rapidly. Ammonia and similar toxins can be difficult to remove from the body because they do not bind well to proteins and are distributed throughout a large volume. Intermittent hemodialysis may be used for initial rapid removal, followed by CRRT, to handle the rebound effect caused by toxin redistribution from the cells to plasma.[12] This combination therapy may also be administered in metformin toxicity due to similar redistribution from the erythrocytes to the vascular space. Inflammatory mediators such as interleukin 1 (IL-1), IL-6, IL-8, and tumor necrosis factor-α may also be removed via convection, which is presumed to help manage sepsis and its resultant multiorgan failure.
Two additional contraindications of IHD are metabolic acidosis and electrolyte abnormalities.[13][14][15][16] CRRT can easily manage hyponatremia by adjusting the composition of the dialysate and replacement fluids or running a parallel dextrose 5% water infusion to achieve the desired sodium. This technique is based on calculations assuming a closed circuit. However, due to the continuous influx and efflux of fluids, close sodium monitoring is needed to allow adjustments to solutions. Hyperkalemia management typically warrants more rapid blood flow, making IHD preferable. However, CRRT may be utilized if the patient is significantly hemodynamically unstable.
Patients on extracorporeal membrane oxygenation (ECMO) commonly develop AKI due to hemodynamic insults. ECMO often necessitates RRT initiation primarily due to fluid overload and electrolyte imbalances. CRRT can be administered using either an integrated CRRT device or a parallel system consisting of separate ECMO and RRT circuits. This technique requires in-depth knowledge of associated complications (eg, pressure changes in the circuit, air entrapment, hemolysis, and thrombocytopenia).[17]
Contraindications
CRRT is contraindicated when treatment outcomes demand a faster resolution than what this intervention can provide. Conditions that may render CRRT less favorable include patient directives against dialysis, vascular access challenges, inadequate expertise or equipment availability, and irreversible liver failure in patients ineligible for liver transplantation.
Equipment
CRRT is performed using a specialized machine comprised of a filtration apparatus. The following is the list of medical products required to perform CRRT:
- Blood purification machine
- Blood warmer
- Filter
- Dialysate
- Replacement fluid
Dialysis and replacement solutions can be lactate- or bicarbonate-based. A great degree of flexibility exists in managing fluid composition due to the availability of sterile pre-mixed bags in the market, eg, Baxter, NxStage, and B.Braun. KDIGO recommends bicarbonate-based solutions in individuals with liver failure or hypoperfusion, as lactate-based solutions may precipitate lactic acidosis in these patients.
Contact between blood and the CRRT circuit components, such as tubing and dialysis membrane, can trigger platelet activation, release pro-inflammatory markers, and stimulate prothrombotic mediators. These events can lead to filter clotting, thereby reducing the effectiveness of the delivered dialysis dose through diminished surface area availability. Anticoagulation is necessary to counteract this phenomenon.
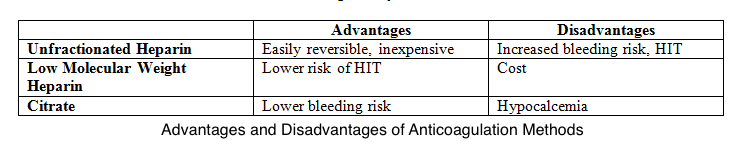
Unfractionated heparin is the most preferred anticoagulant despite the associated risks of heparin resistance, heparin-induced thrombocytopenia, and bleeding. Low molecular weight heparins are less likely to cause heparin-induced thrombocytopenia than unfractionated heparin. However, these agents are more expensive than unfractionated heparin.
Meanwhile, many institutions use regional citrates for anticoagulation per KDIGO guidelines, giving less circuit loss risk than regional and systemic heparin. This alternative also has a reduced bleeding risk compared to systemic heparin but a similar bleeding risk to regional heparin.[18] Infused citrate chelates calcium in the arterial circulation, impairing calcium-dependent clotting factors and inhibiting the coagulation cascade. Target citrate and calcium levels to ensure complete inhibition of coagulation are 3 to 5 mmol/L and less than 0.35 mmol/L, respectively.[19] Both systemic and regional anticoagulation have advantages and disadvantages (see Image. Comparison Between CRRT Anticoagulation Methods).
Personnel
Performing CRRT requires a collaborative team effort before, during, and after the procedure. Expert involvement across various domains, including a critical nephrology specialist and a proficient team of CRRT nurses, can aid in establishing priorities, implementing quality control measures, and enforcing standardized policies.[20][21]
The involvement of specialists external to conventional intensive care staff with expertise in critically ill patients is not new. The following is a list of external groups involved in the interprofessional management of patients on CRRT:
- Respiratory care practitioners
- Nutritional support team
- Clinical pharmacology
- Diagnostic and interventional radiology
- Cardiology
- Rehabilitation and physiotherapy
- Nursing staff training in the ICU [22]
These healthcare professionals bring unique skills contributing to comprehensive patient care and may collaborate with ICU staff to optimize patient outcomes.
Preparation
Effluent Dose
The recommended average delivered effluent dose is 20 to 25 mL/kg/hr for patients with AKI needing CRRT based on the Veterans Affairs/National Institutes of Health Acute Renal Failure Trial Network Study and Randomized Evaluation of Normal versus Augmented Level Replacement Therapy Study. No benefit is seen above 25 mL/kg/hr. However, the prescribed dose does not equal the delivered dose due to various factors, such as circuit downtime during procedures, imaging tests, filter clotting, or fluid bag replacement.
Net Ultrafiltrate
Significant heterogeneity exists in practice regarding the net fluid removal goal and rate.[23] Fluid removal has to be individualized and is subject to change. The general recommendation is to stay below 1.5 to 2.0ml/kg/hr.
Blood Flow Rate
A minimum blood flow of 150 mL/min can be used to maximize clearance.[24] This value maximizes clearance efficiency while maintaining hemodynamic stability during CRRT.
Technique or Treatment
CVVH
This method utilizes hydrostatic pressure across a semipermeable membrane for ultrafiltration using convection to filter solutes. Higher- and lower-molecular-weight solutes are transported with equal efficacy until the solute molecular radius exceeds the membrane pore size. A high ultrafiltration rate is required to allow sufficient solute clearance. Thus, substitute fluid is necessary to replace the filtered fluid. The substitute or replacement fluid can be introduced before the filter, after, or both. However, introducing it after the filter may lead to hemoconcentration, increasing the risk of sludging and circuit occlusion. This occurrence is represented by the filtration fraction, which should not exceed 20% to 25% to avoid clotting and loss of the circuit.
Filtration fraction: Total filtration rate/plasma flow rate + prefilter replacement fluid rate
Introducing replacement fluid before the filter can dilute the blood and decrease the filtration fraction, decreasing clotting risk, but this may reduce effective solute clearance.
CVVHD
This technique relies on diffusion across a transmembrane concentration gradient established by the flow of dialysate fluid countercurrent to the blood. Smaller solutes like potassium, urea, and creatinine pass more readily across the membrane than larger solutes. Ultrafiltration rates are comparatively lower than in CVVH.
CVVHDF
This modality combines convection and diffusion filtration methods at high ultrafiltration rates, using dialysate and replacement fluid to remove small and middle-sized molecules from the blood efficiently. Dialysate composition or substitution fluids can be customized to achieve the desired plasma composition, which is particularly beneficial in electrolyte derangements and lactic acidosis, where a bicarbonate buffer may be utilized. CRRT, used solely for volume management without solute removal, is termed "slow continuous ultrafiltration" or "SCUF."
Calcium is often not incorporated in the available premixed replacement fluid bags. Therefore, patients require a separate calcium infusion to replete what is lost in the circuit, with the dose adjusted according to the ionized calcium level.
Troubleshooting the CVVH Machine
Vascular access can be maintained by ensuring adequate blood flow. Various alarms on the machine can help identify CVVH circuit problems. A high-venous or low-arterial pressure alarm may signify kinking, clotting, or blockage of blood inflow due to inlet holes pressing against the blood vessel wall. This problem may be remedied with either catheter rotation or replacement, heparin or tissue plasminogen activator infusion followed by rinsing, or a limb switch.
A low-arterial pressure alarm may indicate hypovolemia. A disconnection alarm typically indicates either line separation or, less commonly, device disconnection from the patient. This alarm can usually be overridden if no physical issue is detected. Often, the culprit is a forgotten clamp. In some instances, adjusting the blood flow rate relative to catheter performance may be necessary.
The session must be discontinued if air is present in the circuit or arterial access. Small carbon dioxide bubbles may form from bicarbonate in the hemofiltration bags. The manual provides degassing instructions. Notably, a fluid balance error can occur if the dialysate or replacement fluid bags are not hung correctly, necessitating repositioning.
Complications
As in every procedure, CRRT has risks that should be communicated to the patient or family during treatment planning. First, the risks associated with intravascular lines include hemorrhage, arteriovenous fistula formation, infection, and thrombosis. The risks of the therapy itself include electrolyte disturbances, clearance of trace elements or antibiotics, hypothermia, and hypotension.
Although hypotension occurs less commonly than in IHD, blood pressure changes may occur if the net ultrafiltration rate exceeds the intravascular refilling rate. The electrolyte and acid-base status should be monitored every 6 to 12 hours when starting CRRT. The interval may be increased to 12 to 24 hours if the condition remains stable after the first 24 to 48 hours. As discussed above, the exception is when using citrate as regional anticoagulation, which requires frequent ionized calcium level monitoring.
Hypocalcemia, hypokalemia, and hypophosphatemia are the commonly managed electrolyte imbalances with CRRT. The degree of deficiency depends on the CRRT dose delivered. In the Randomized Evaluation of Normal versus Augmented Level Replacement Therapy, hypophosphatemia was a reported electrolyte abnormality in 65% of patients undergoing high-intensity CRRT. This electrolyte deficiency can be managed with intravenous or oral repletion, enteral feed fortification, or the use of a phosphate-containing dialysate or replacement solution.[25]
Medication clearance during CRRT is variable. Thus, the doses of required drugs must be checked when administering CRRT. Trough concentrations of these medications determine their bactericidal or bacteriostatic effectiveness. The most clearance-susceptible during CRRT are water-soluble antimicrobials, aminoglycosides, and β-lactam antibiotics.[26] Many patients who meet CRRT indications are septic, meaning that appropriate antibiotic dosage is vital. CRRT also often results in amino acid, micronutrient, and water-soluble vitamin loss. Patients are likewise often in a substantial negative nitrogen balance. Appropriate caloric and protein intake with supplementation of water-soluble vitamins should be ensured. Meanwhile, the risks associated with using an extracorporeal circuit include hypersensitivity to the circuit, air embolization, and blood loss during filter or circuit changes.
Clinical Significance
When to Initiate CRRT
Many factors should be considered when deciding to initiate CRRT. The most important are the illness's severity and the procedure's necessity. AKI's gravity and its trajectory may be used to assess disease severity. Other factors include the presence of electrolyte and acid-base disorders, evidence of fluid overload, and other significant organ dysfunction requiring renal support for recovery. The timing of initiation is up for debate without specific indications.
Early initiation may allow for early correction of electrolyte abnormalities, volume status, and azotemia before significant disturbances occur but necessitates careful consideration of potential complications. AKI trajectory is challenging to predict, so isolating patients likely to have a persistent AKI is not always reliable.[4] Factors that may aid the assessment include the likelihood of AKI reversibility, the presence of oliguria, and the renal insult's nature and timing. Thus, the timing of initiation varies from patient to patient without a clear consensus.
The timing of RRT initiation in severe AKI remains controversial due to the unpredictability of kidney function trajectory. Hoste et al's multicenter study found that implementing a furosemide stress test aided in identifying patients with severe AKI at high risk of requiring RRT. Specifically, among nonresponders to intravenous furosemide, characterized by a urine output of less than 200 mL in 2 hours, a substantial proportion (78%) ultimately necessitated RRT initiation.[27] Although no mortality difference was noted, the study lacked power, necessitating further investigation.
When to Discontinue CRRT
No standard approach exists to discontinuing CRRT or transitioning to IHD. Liya et al's 2021 meta-analysis determined, though with limited evidence, that urine output (p = 0.0000), serum creatinine (p = 0.008), serum potassium (p = 0.02), serum bicarbonate (p = 0.01), pH value (p = 0.03), urinary urea (p = 0.002), and urinary creatinine (p = 0.02) were significantly associated with weaning success.[28] Therefore, these parameters must be monitored daily to determine when CRRT can be weaned after achieving sustained hemodynamic stability. Similarly, Li et al's meta-analysis also determined that chronic kidney disease, CRRT duration, and urine output at the time of CRRT discontinuation were predictive of short-term successful CRRT weaning.[29] While some may be weaned off CRRT, many may still require dialysis but can be transitioned to IHD when monitoring for renal function improvement.
Enhancing Healthcare Team Outcomes
CRRT requires an interprofessional healthcare team approach to be provided safely and efficiently. The modality involves the collaboration of specialties, including critical care, nephrology, and neurology, on essential elements such as the timing of initiating a clearance mode, solute and fluid removal targets, and anticoagulation strategies. All clinicians involved in the case, including nurse practitioners and physician assistants, must contribute to care. Nursing is vital during CRRT as nurses have the most exposure to the vascular access site, the CRRT circuit, and the patient.
Nursing staff and patient care technicians should be aware of complications to allow timely intervention and communication with the clinician team regarding the event. Additionally, pharmacists and nutritionists are vital to ensure proper medication doses and nutrition while on CRRT. Pharmacists should offer insights on CRRT's impact on the patient's medication regimen and therapeutic outcomes, collaborating with the team to adjust drug therapy as needed. With this team approach and adequate education for each member, CRRT may be delivered as safely and effectively as possible.[30]
Nursing, Allied Health, and Interprofessional Team Interventions
The following actions are expected from the interprofessional team:
- Collaboration between clinician teams
- Defining CRRT's primary goal
- Ensuring adequate access, machine, and anticoagulation to maintain high-functioning CRRT with minimal disruptions
- Ensuring appropriate nutrition support
Nursing, Allied Health, and Interprofessional Team Monitoring
The following variables must be monitored closely while patients are on CRRT:
- CRRT prescription and response
- Aappropriateness of medication dosing
- Lab results and circuit performance to detect CRRT complications