Continuing Education Activity
A chylothorax is the accumulation of chyle in the pleural space. This is most commonly seen following traumatic disruption of the thoracic duct and is typically diagnosed based on the milky appearance of fluid due to high-fat content. Most patients with chylothoraces will require surgical exploration of the thoracic duct. This activity reviews the evaluation and management options for patients with chylothoraces and highlights the role of the interprofessional team in caring for these patients.
Objectives:
- Describe the pathophysiology of a chylothorax.
- Summarize how to evaluate for a chylothorax.
- Outline the treatment options for a chylothorax.
- Explain a well-coordinated interprofessional team approach to provide effective care to patients with chylothorax.
Introduction
Chylothorax is the accumulation of chyle in the pleural cavity. The word chyle is derived from the Greek word “Chylos,” which means juice. Chyle is the milky bodily fluid formed in the lacteal system of the intestine. The small and medium-chain triglycerides consumed in the diet are easily broken to free fatty acids by the intestinal enzymes and readily absorbed into the portal circulation. However, the large molecules of complex long-chain triglycerides cannot be broken down by the intestinal lipases. They combine with phospholipids, cholesterol, and cholesterol esters to form chylomicrons in the jejunum. These large molecules then get absorbed into the lymphatic system of the small intestine to form the chyle. The lymphatic drainage system of the intestine will be joined by the lymphatic drainage from the lower extremities to form the thoracic duct system, which ultimately drains in the system circulation. If there is a breach of the integrity of the thoracic duct, the milky lipid-rich chyle leaks to the surrounding structures. Chylothorax is formed when the chyle leaks into the pleural cavity due to damage to the thoracic duct. As the normal chyle production is around 2.4 liters per day, a considerable amount of chyle can accumulate in the pleural cavity in a very short period. Chylothorax was first described in the 17th century by Dr. Bartloet and, in the last decade, has received special attention due to the innovation of new management strategies with favorable outcomes.[1][2][3][4]
Etiology
The etiology of chylothorax can be classified into three broad categories, spontaneous (non-traumatic), traumatic, and idiopathic. Historically, non-traumatic chylothorax was the more common cause for chylothorax, accounting for two-thirds of all cases. Recently, traumatic chylothorax, particularly postoperative chylothorax, accounts for more than 50% of all cases described in the literature.[5][6][4]
Non-traumatic chylothorax can be due to the following:
- Congenital chylothorax can be seen as an isolated condition or in association with other lymphatic abnormalities like lymphangiectasis, lymphangiomatosis, tuberous sclerosis, congenital heart disease, or chromosomal abnormalities such as trisomy 21 or Turner syndrome.
- Neoplastic chylothorax is the most common cause of non-traumatic chylothorax. Various cancers like lymphoma, chronic lymphoid leukemia, lung cancer, esophageal cancer, or metastatic carcinoma have been implicated in chylothorax. Interestingly, there is a relative decrease in the incidence of chylothorax in patients with lymphoma in recent times. This is likely due to early diagnosis and treatment of lymphoma, avoiding the late complication of chylothorax.
- Infectious chylothorax is mostly seen in developing countries as a complication of tuberculous lymphadenitis. Other infections are known to cause chylothorax are aortitis, histoplasmosis, and filariasis.
- Rare causes of chylothorax reported in the literature are due to cattleman disease, sarcoidosis, Kaposi sarcoma, yellow nail syndrome, Noonan syndrome, Down syndrome, Waldenstrom macroglobulinemia, macroglobulinemia, amyloidosis, venous thrombosis, thoracic radiation, goiter. All these reported diseases will lead to obstruction or destruction of the thoracic duct, causing chylothorax. Even parental nutrition therapy is implicated in chylothorax. Rapid infusion of total parenteral nutrition containing a high concentration of triglycerides can overwhelm the draining capacity of the thoracic duct leading to leakage of chyle to this surrounding pleural space and cause chylothorax.
Traumatic chylothorax occurs due to disruption of the thoracic duct anywhere in its course in the mediastinum. They can occur as a complication of the surgical procedure or can follow blunt or penetrating trauma to the chest.
- Postoperative chylothorax constitutes the most common cause of chylothorax in modern medicine. The risk of postoperative chylothorax depends on the type of surgery done. Esophagostomy carries the highest risk of 5% to 10% postoperative chylothorax, followed by lung resection with mediastinal lymph node dissection with 3% to 7% risk. Other surgeries like mediastinal tumor resection, thoracic aneurysm repair, sympathectomy, any surgeries in the lower neck and mediastinum carry a risk of chylothorax. Ingestion of milk before the surgery can lead to better visualization of the thoracic duct in the surgical field by causing whitish discoloration of the thoracic duct. It is proposed on of the preoperative steps to prevent postoperative chylothorax, although this has not been formally studied. Nonsurgical posttraumatic chylothorax is also described after central line placement, pacemaker implantation, embolization of the pulmonary arteriovenous malformation.
- Blunt trauma to the chest or thoracic spine can disrupt the thoracic duct without any obvious injury to the surrounding structure leading to chylothorax. Chylothorax is also described after blasting injuries. Chylothorax is also described following a trivial injury like coughing and sneezing.
- Penetrating injuries of the chest like gunshot injury and stab injury can directly damage the thoracic duct leading to chylothorax.
Idiopathic causes account for nearly 10% of all cases of chylothorax. Chylothorax is considered to be idiopathic after extensive investigation does not reveal any known cause for it. Most of these idiopathic cases are related to undiagnosed malignancy.
Epidemiology
This is a rare condition that may develop as a complication of thoracic and esophageal and thoracic surgery and hematologic malignancies. Chylothorax has no predilection for gender or age. The prevalence after various cardiothoracic surgeries is 0.2% to 1%. Mortality and morbidity rates are approximately 10%.
Pathophysiology
Anatomy
It is essential to understand the anatomy of the lymphatic system to understand the chylothorax. The lymphatic system is composed of lymphatic vessels, lymph nodes, and the cisterna chyli. The thoracic duct is the main collecting vessel of the lymphatic system. It drains three-quarters of the lymph in the body into the venous bloodstream. The lymphatic system, which connects the lymphatics of various organs, is an important network for the circulation of fluid throughout the body. The lymph from the lower limb finally terminates at the para-aortic nodes. They join with the lymph from the viscera of the pelvis and form bilateral lumbar trunks. The intestinal trunks consist of large lymphatic vessels that receive lymph from the stomach, intestine, pancreas, and spleen. Cisterna chyli is a triangular dilation of the lymphatic system located in the retroperitoneum, just posterolateral to the abdominal aorta adjacent to the second lumbar vertebra. The thoracic duct starts from the cisterna chyli and is located at the level of the second or third lumbar vertebra. It enters the thorax through the aortic hiatus of the diaphragm and ascends in the posterior mediastinum. It is located between the descending thoracic aorta on the left and the azygos vein on the right. At the level of the fifth thoracic vertebra, the thoracic duct inclines toward the left side to enter the superior mediastinum. It ascends behind the aortic arch and the thoracic part of the left subclavian artery, between the left side of the esophagus and the left pleura, to the thoracic inlet. Finally, the duct terminates by opening into the junction of the left subclavian and jugular veins. However, the duct may open into either of the great veins near the junction, or it may divide into a number of smaller vessels before termination. In adults, the thoracic duct varies in length from 38 cm to 45 cm. It is approximately 3 mm to 5 mm in diameter at its commencement but diminishes in caliber at the mid-thoracic level and then slightly dilates before its termination. So, a breach in the integrity of the thoracic duct anywhere in its course in mediastinum will lead to seepage of chyle in the pleural cavity leading to chylothorax.
Composition of Chyle
Chyle is made primarily of chylomicrons, an aggregate of long-chain triglycerides, cholesterol esters, and phospholipids. It is also rich in lymphocytes, primarily T lymphocytes as the major cellular component with concentrations that range from 400 to 6800 cells. Chyle as similar electrolyte concentration plasma but is rich in immunoglobulins with fat-soluble vitamins.
History and Physical
The clinical features of chylothorax depend upon the cause. Small chylothorax can be asymptomatic and is detected incidentally. Patient with large chylothorax usually presents with signs and symptoms caused by the mechanical effect of compression on lung expansion. Progressive breathlessness decreases exercise capacity; chest pressure is common presenting complaint. Fever and chest pain is usually absent. The patient can accumulate a large amount of chylothorax without any complaints if the fluid recommendation is gradual and the respiratory system gets acclimatized to it. Post-traumatic chylothorax can present up to 10 days after the inciting trauma. In the post-surgical patient’s, the chylothorax me the first detected as a pleural effusion or by persistent drainage from the indwelling chest tubes.
On physical examination, findings of decreased breath sounds and dullness to percussion may be present depending on the size and location of fluid. Eighty percent of chylothorax cases are unilateral. Due to the location of the thoracic duct, the right side is more common than the left, accounting for two-thirds of the total cases.
Evaluation
Further evaluation of chylothorax depends on the suspected cause and the availability of resources.
Chest X-ray
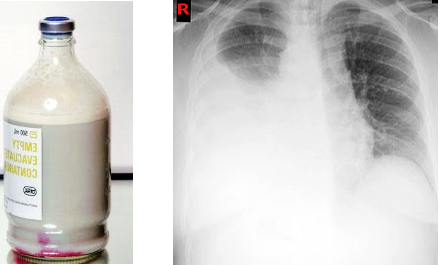
Chest radiographs routinely done to evaluate dyspnea, particularly in postoperative and traumatic patients, can detect unilateral pleural effusion. Chylothorax appears as a homogenous density obligating the costophrenic and cardio phrenic angle. A routine chest x-ray cannot differentiate chylothorax from other causes of pleural effusion.
Thoracic Ultrasound
Ultrasound is being increased used for the evaluation of patients with pleuropulmonary pathology. Like other effusions, it appears as an isodense echoic region without any septation or loculation. However, ultrasound cannot differentiate chylothorax from other causes of pleural effusion.
Chest CT Scan
CT scan is more sensitive than chest x-ray and ultrasound for the diagnosis of chylothorax. Routine CT chest can show cisterna chyli in around 2% of cases. Due to the high amount of fat content, it is seen as a low-attenuation tubular area in the posterior mediastinum. CT scan also may show the cause of chylothorax like mass lesions or obstructive lesion in the posterior mediastinum, thoracic malignancy, or evidence of trauma.
MRI
MRI of the chest can show cisterna chyli in 100% of the cases and can be used for better evaluation of chylothorax. However, as MRI of the chest is not an optimal investigation for the thoracic pathology, it is rarely used in clinical practice.
Conventional Lymphangiography
Lymphangiography is a technique used to delineate the lymphatic system. In modern medicine, this test is rarely used due to the availability of less invasive alternatives that are equally precise. In this technique, dye like methylene blue that stains the lymphatics is injected into the web spaces between the toes. A longitudinal or transverse cutaneous incision at the base of the first metatarsal bone is made to expose a lymphatic vessel with blue staining after dissection of the surrounding tissue. The isolated lymphatic vessel is then cannulated using a 30 gauge needle. After accessing the lymphatic vessel, 10 mL of Lipiodol is slowly injected at the rate of 0.2 mL/min to 0.4 mL/min. Serial fluoroscopic spot images are obtained upwardly every 5 to 10 minutes over the course of the injected Lipiodol. If Lipiodol does not reach the area of interest, normal saline at the same rate can be injected to push Lipiodol further into the area of interest. This can reliably show any leakage in the thoracic duct leading to chylothorax. A further modification of this called nodal lymphangiogram has been developed in recent times. A targeted lymph node is selected, and ethiolised oil is injected into the cortex of the lymph node at a rate of 1 ml per min for a total of 10 ml. Serial spot radiographs of the pelvis, abdomen, and thorax are then acquired to follow the progression of the dye. This Intranodal lymphoscintigraphy is sensitive, technically easier, and also has fewer complications. Lymphogram can be therapeutic in up to 40% of cases. The high-density oil used in the procedure can close in leak during the procedure.
Nuclear Lymphoscintigraphy
This technique has been used more commonly than traditional lymphangiography to delineate the lymphatic system. In this procedure, Tc99m labeled human diethylenetriaminepentaacetic acid (HAS-DTPA) is injected into the subcutaneous lesions of the dorsum of the foot bilaterally. Sequentially anterior and posterior images of the chest are obtained using a gamma camera to identify the leak. This technique can be combined with integrated low-dose CT scan with single-photon emission computed tomography combined to get the more accurate SPECT/CT images. Radio nuclear localization using lymphoscintigraphy has been shown to correlate with traditional lymphangiography and surgical localization of leaks.
Laboratory Testing
Thoracentesis and analysis of the fluid is the diagnostic study of choice for chylothorax
All fluid samples should be sent for white blood cell count, differential, glucose, Lactic dehydrogenase, total protein level, cytology, microbiology smear, and culture. If chylothorax is suspected based on the color of the fluid, then additional tests like Ph, total triglycerides levels, and cholesterol levels should be sent.
Color
Based on the amount of fat content in chylothorax, the appearance of fluid can (white) milky, serous, or serosanguineous. The absence of a milky appearance does not exclude a chylothorax. In chylothorax, after the centrifugation of the pleural fluid, the supernatant is opaque in chylothorax and cholesterol effusions and clear in empyema. In this case the milkiness is caused by suspended leukocytes and debris.
Chyle is rich in lymphocytes accounting for 80% of all the cells. The lymphocytes are predominately polyclonal population of T cells.
Lipid Analysis
Measurement of the pleural fluid triglyceride content is key to the diagnosis of suspected chylothorax. Unlike in any other body fluids, chylothorax is very rich in large chain fatty acids absorbed from the small intestine. The pleural fluid triglyceride concentration greater than 110 mg/dL confirms the diagnosis of chylothorax. However, 15% of chylothorax are known to have triglyceride concentrations less than 110 mg depending on the time of the last meal and the fat content of the diet. If clinical suspicion of chylothorax is high, then lipid electrophoresis of the pleural fluid should be done. Detection of chylomicrons in the pleural fluid by lipoprotein electrophoresis confirms chylothorax. Typically, the total cholesterol level in a chylothorax is less than 200 mg/dl.
Others Compositions
The other fluid composition is similar to plasma. Chylothorax is usually alkaline, with PH ranging from 7.4 to 7.8.
Pseudo-chylothorax or chyliform effusion. Effusions with a gross appearance similar to chylothorax, milky white are called pseudo-chylothorax. These are less common than the classical chylothorax and have been reported being in long-standing exudate pleural effusion of various causes. They contain a high concentration of cholesterol, giving them that characteristic milky or white appearance. In long-standing exudative pleural effusion, the cell undergoes slices releasing the cholesterol from the cell membrane into the fluid, which gets trapped in the pleural cavity. However, unlike classical chylothorax, they do not contain chylomicrons or long-chain fatty acids. Pseudo- chylothorax is commonly associated with tuberculosis and chronic rheumatoid pleural effusion due to the high cell concentration. Other causes described and literature are yellow nail syndrome and paragonimiasis. The cholesterol concentration in pseudo-chylothorax is typically more than 200 mg; triglycerides level is less than 110 mg/dL, and cholesterol to triglycerides ratio is always more than 1. Cholesterol crystals may be visible in dried slides from pleural fluid when viewed under polarized light and appear rectangle plates with notched edges. The presence of cholesterol crystals is virtually diagnostic of pseudo-chylothorax.
Treatment / Management
Management
The appropriate management of chylothorax depends on the cause and includes one or more of many interventions like dietary modification, pleurodesis, and thoracic duct ligation. Recently, the use of somatostatin/octreotide, midodrine, and sirolimus to prevent chyle formation has been used. New surgical techniques like pleurovenous or pleuroperitoneal shunting and thoracic duct embolization have been used with success. Most patients benefit from a staged care plan, from least invasive options to more invasive techniques.[7][8][9][10]
Dietary therapy
As chylous fluid is formed the long-chain fatty acids; decreasing or eliminating these fatty acids from the diet will lead to decreased chyle drainage spontaneously closure of leak. Diet with less than 5 kcal of fat per meal is used in this technique. This can effectively reduce chyle formation, but over long periods, it will lead to fat deficiency and malnutrition. In fact, venous fat hemorrhage can remedy some of the shortcomings of this therapy. Small chain and medium-chain fatty acids can be provided in a diet, and long-chain fatty acids can be supplemented intravenously.
Thoracentesis
Intermittent therapeutic thoracentesis or the use of an indwelling catheter for home drainage is typically used in the initial management of non-traumatic and nonsurgical traumatic effusions to relieve dyspnea caused by the pleural fluid. This technique can be effectively used if there is a gradual recommendation of her pleural fluid. A chest tube can be used in postsurgical chylothorax. Prolonged drainage of pleural fluid comes with a risk of significant malnutrition and loss of immunoglobulins, putting the patient at risk of infections. Hence continuous pleural fluid drainage should typically be restricted to less than 2 weeks. Surgical intervention is recommended if the pleural fluid drainage exceeds 1.5 L per day.
Pleurodesis
This should be reserved for patients who continue to reaccumulate fluid despite dietary modification and repeated thoracenteses. Pleurodesis can be accomplished by tall installation of a chest tube drainage by video-assisted thoracic surgery with talc insufflation. In some cases, this procedure can remedy up to 80% of chylothorax. Concomitant ligation of the thoracic duct to prevent further chyle formation during surgical pleurodesis is also done with high success.
Thoracic Duct Ligation
This invasive procedure is done using video-assisted thoracic surgery and can be useful in patients where dietary modification and pleurodesis has failed. Lymphedema is a known complication of thoracic duct ligation but usually resolves after several months due to collateral lymphatic venous communications.
Thoracic Duct Embolization and Disruption
Percutaneous catheterization and embolization are needle disruption of the thoracic duct, and cisterna chyli, along with prominent retroperitoneal lymphatic ducts, have been used increasingly in both traumatic and nontraumatic chylothorax. Initial pedal lymphangiogram follows fluoroscopic visualization of the large retroperitoneal lymphatics, which has been accessed by transabdominal percutaneous needle cannulation. After cannulation of the cisterna chyli, the catheter is advanced with thoracic duct installation of contrast to localize the leak. The affected thoracic segment is then embolized with coils and surgical clues.
Emerging Therapies
Somatostatin and octreotide can decrease the gastric, pancreatic, and biliary secretions and reduce the total flow of gastric lymphatics. Because of the reduction of chyle formation and flow rates, they can spontaneously remedy the leak in the thoracic duct. This technique is reported to be useful in many cases of spontaneous chylothorax, congenital chylothorax, postoperative chylothorax, and chylothorax due to malignancy. The optimal dose and duration of treatment are not clear.
Sirolimus used for the effect to the treatment of lymphangiomyomatosis is also known to decrease the incidence of chylothorax in these sets of patients.
Pleuroperitoneal or pleural venous shunt. Shunting of chylous pleural fluid into the venous system or peritoneal cavity can also resolve chylothorax. Two types of pleuroperitoneal shunts are available. Denver pleuroperitoneal shunt is an active pump that requires manual pumping, and Le Veen's pleuroperitoneal shunt is a passive pump. The main advantage of the shunt procedure is the recycling of the nutritionally rich chyle to the body. Pleural venous shunting of chylous fluid from the pleural space to the subclavian jugular vein has been performed successfully in patients with yellow nail syndrome and postsurgical chylothorax.
Differential Diagnosis
- Exudative pleural effusion
- Malignant pleural effusion
- Parapneumonic pleural effusions
Pearls and Other Issues
Chylothorax is caused by disruption of the thoracic duct and distributor resulting in chyli (lymphatics fluid of interstitial region) into pleural space. This can be seen in many conditions, and trauma and malignancy are the leading causes. The diagnosis based on pleural fluid can be made by demonstration of triglycerides of more than 110 mg/dL or the presence of chylomicrons. Various surgical and nonsurgical management strategies can be used for the treatment of chylothorax. Currently, somatostatin analogs are effectively used for its treatment.
Enhancing Healthcare Team Outcomes
There are many causes of chylothorax, and its management is complex. Thus, the condition is best managed by an interprofessional team that consists of a pulmonologist, thoracic surgeon, dietitian, internist, and an intensivist. Initially, the treatment is conservative, but if the leak does not stop, surgery is required. New surgical techniques like pleurovenous or pleuroperitoneal shunting and thoracic duct embolization have been used with success. Most patients benefit from a staged care plan, from least invasive options to more invasive techniques. Overall, the outcome for chylothorax from benign causes is good, but in cases of malignancy, the outcomes are poor.[11] [Level 5]