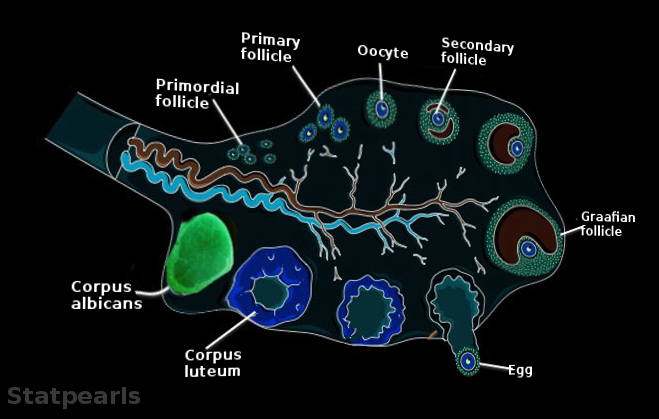
[2]
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP, Williams CJ, Erickson GF. Morphology and Physiology of the Ovary. Endotext. 2000:():
[PubMed PMID: 25905186]
[3]
Duncan WC. The human corpus luteum: remodelling during luteolysis and maternal recognition of pregnancy. Reviews of reproduction. 2000 Jan:5(1):12-7
[PubMed PMID: 10711731]
[4]
Mihm M, Gangooly S, Muttukrishna S. The normal menstrual cycle in women. Animal reproduction science. 2011 Apr:124(3-4):229-36. doi: 10.1016/j.anireprosci.2010.08.030. Epub 2010 Sep 3
[PubMed PMID: 20869180]
Level 3 (low-level) evidence
[5]
Perheentupa A, Huhtaniemi I. Aging of the human ovary and testis. Molecular and cellular endocrinology. 2009 Feb 5:299(1):2-13. doi: 10.1016/j.mce.2008.11.004. Epub 2008 Nov 18
[PubMed PMID: 19059459]
[6]
Galvão AM, Skarzynski D, Ferreira-Dias G. Luteolysis and the Auto-, Paracrine Role of Cytokines From Tumor Necrosis Factor α and Transforming Growth Factor β Superfamilies. Vitamins and hormones. 2018:107():287-315. doi: 10.1016/bs.vh.2018.01.001. Epub 2018 Feb 9
[PubMed PMID: 29544635]
[7]
Rojas J, Chávez-Castillo M, Olivar LC, Calvo M, Mejías J, Rojas M, Morillo J, Bermúdez V. Physiologic Course of Female Reproductive Function: A Molecular Look into the Prologue of Life. Journal of pregnancy. 2015:2015():715735. doi: 10.1155/2015/715735. Epub 2015 Dec 1
[PubMed PMID: 26697222]
[8]
Hariri LP, Bonnema GT, Schmidt K, Winkler AM, Korde V, Hatch KD, Davis JR, Brewer MA, Barton JK. Laparoscopic optical coherence tomography imaging of human ovarian cancer. Gynecologic oncology. 2009 Aug:114(2):188-94. doi: 10.1016/j.ygyno.2009.05.014. Epub 2009 May 29
[PubMed PMID: 19481241]
[9]
Mesen TB, Young SL. Progesterone and the luteal phase: a requisite to reproduction. Obstetrics and gynecology clinics of North America. 2015 Mar:42(1):135-51. doi: 10.1016/j.ogc.2014.10.003. Epub 2015 Jan 5
[PubMed PMID: 25681845]
[10]
Millet J, Much M, Gunabushanam G, Buza N, Schwartz PE, Scoutt LM. Large ovarian calcifications from an unresorbed corpus albicans. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2012 Sep:31(9):1465-8
[PubMed PMID: 22922629]