Continuing Education Activity
This activity describes the use of gastric emptying scintigraphy in the evaluation of gastric motility and related disorders. It discusses its role in the assessment of patients with symptoms of gastroparesis or abnormal gastric emptying and discusses the potential use of this procedure to improve patient care. Proper patient preparation and exam technique are reviewed as well as interpretation criteria. The activity highlights the study and its use by a diverse specialty of providers in managing patients with dyspepsia.
Objectives:
Review the anatomical structures pertinent to gastric emptying and their individual roles.
Outline the preparation and examination technique used in gastric emptying scintigraphy.
Describe the indications and contraindications to gastric emptying scintigraphy.
Summarize interprofessional team strategies for improving care coordination and communication to advance the assessment of gastric motility disorders and improve outcomes.
Introduction
The first use of nuclear medicine to evaluate gastric motility was performed in 1966 by Dr. Griffith and colleagues of Cardiff, Wales, using a breakfast meal labeled with Chromium-51.[1] By measuring the amount of radioactivity in the stomach (gastric counts) at various time points, they could directly determine the volume of a meal remaining in the stomach and thus determine the rate of gastric emptying (GE). Since then, the modern version of the exam, known as gastric emptying scintigraphy (GES) has become a common diagnostic tool in the assessment of patients with various functional gastrointestinal disorders.
Other tests used to measure GE include breath testing and wireless pH capsules. Breath testing is performed using a standardized meal including Spirulina labeled with Carbon-13. The meal passes through the stomach, into the duodenum where it is absorbed, metabolized in the liver and exhaled by the lungs where it is measured. As transit of the meal through the stomach is the rate-limiting step in the process, the test serves as an indirect measurement of GE, assuming normal bowel, liver, and pulmonary function. The wireless pH capsule test is performed by administering a capsule in conjunction with a nutrient bar. The capsule is monitored by a belt worn by the patient and transit from the stomach to small bowel is detected by a sudden increase in pH, denoting transition from the acidic stomach to the alkaline duodenum.
Given its noninvasive nature and physiologic methodology compared to these other tests, scintigraphy has become the prevailing means by which to measure gastric emptying (GE).
Anatomy and Physiology
The stomach is in the left upper quadrant (LUQ) of the abdomen and is comprised of four functional components: the fundus (proximal), body (mid) and antrum (distal) as well as the pylorus. The fundus is posterolateral and the antrum is anteromedial with the long axis of the stomach oriented obliquely, from the LUQ to the epigastric region. The fundus has two functions. It relaxes with the entrance of solids and liquids, a process called accommodation, and then contracts to provide a pressure gradient which moves the meal distal. The body is a reservoir for mixing of ingested material and serves as the pacemaker for the stomach. The antrum is essential in the handling of solids through a process called trituration, grinding the food into 1 to 2 mm particles through repetitive contractions. Once it reaches this threshold size, the pylorus, which serves to control transit of ingested material out of the stomach, will allow it to pass into the duodenum.[2]
Indications
Gastric emptying scintigraphy (GES) is typically obtained to assess for gastroparesis in patients with post-prandial symptoms of nausea, vomiting, abdominal pain, and/or early satiety. GES can also provide important information in patients with esophageal reflux unresponsive to therapy or in diabetics with poor glycemic control to confirm or exclude delayed gastric emptying as a contributing factor in a patient’s poor response to therapy.[3] Additionally, GES is beneficial in the evaluation of patients with colonic inertia being considered for colectomy since individuals with concurrent delayed gastric emptying have a much lower response rate to surgery than those with normal GE.[4]
More recently, GES has been used to evaluate for rapid gastric emptying, which can be seen early in the course of diabetes as well as with cyclic vomiting syndrome, a disorder manifested by recurrent episodes of nausea, vomiting, and lethargy.[5]
Contraindications
- Allergies to the recommended meal
- Hyperglycemia in diabetics (blood glucose greater than 250 to 275 mg/dL)[6]
Equipment
The components of a meal (size, digestibility, calories and nutrient content) all affect the rate of gastric emptying. Solids and fats empty more slowly, whereas liquids, proteins, and carbohydrates empty more rapidly.[7][8][9] Until recently, no standard existed for the meal used in GES. Different methodologies were used at different imaging clinics (orange juice, cereal with milk, oatmeal, scrambled eggs, chicken liver), and as a result, they often had different normal values. This was of concern to clinicians because study results from separate imaging facilities made interpretation and comparison of results problematic. As a result, in 2007 an expert panel of gastroenterologists and nuclear medicine physicians met to decide on consensus standards for gastric emptying scintigraphy. These recommendations were published in 2008.
The standardized meal described in the GES guideline is a solid meal consisting of 0.5 to 1.0 mCi of 99mTc-sulfur colloid scrambled with 120 grams of liquid egg whites (Egg Beaters or generic), 2 slices of white toast, 30 grams of strawberry jelly, and 120 mL of water.[6] It is recommended that this exact meal be utilized for all adult solid gastric emptying scintigraphy studies. The departure of the test meal from this standard precludes accurate comparison to validated normal values and thus, may factitiously alter the diagnosis of normal versus abnormal gastric emptying.
To date, the consensus guidelines only address gastric emptying in regards to a solid meal. However, liquids and solids empty differently from the stomach. Solids generally show early fundal localization (via accommodation) while liquids distribute quickly throughout the stomach. Also, given that liquids do not undergo trituration (an antral function), they empty predominantly via the control of the fundal pressure gradient. This difference may result in some patients with isolated mild-to-moderate fundal dysfunction not being accurately identified on the standard solid gastric emptying study. To overcome this potential inadequacy of GES, additional research has been done regarding the use of a liquid meal. One of the most widely accepted standards was developed by Ziessman and colleagues at Johns Hopkins, using a non-nutrient meal comprised simply of 300 mL water labeled with 0.2 mCi of Tc-99m sulfur colloid or Indium-111 DTPA.[10][11]
Personnel
A nuclear medicine technologist performs GES exams under the supervision of a nuclear medicine physician or nuclear radiologist.
Preparation
Proper patient preparation is critical to performing an accurate and reliable GES.
- Prokinetic agents such as metoclopramide, erythromycin, tegaserod, and domperidone should be discontinued for 2 days before the study unless the test is performed to assess the efficacy of these medications.
- Medications that delay gastric emptying should also be discontinued for 2 days before the exam. These include opiates (e.g., morphine, codeine, and oxycodone) and antispasmodic agents such as atropine, dicyclomine, loperamide, and promethazine.
- Patients should not eat or drink for a minimum of 4 hours before the study. It is typical for the patient to take nothing by mouth starting at midnight and then undergo the exam in the morning.
- Insulin-dependent diabetic patients should bring their insulin and glucose monitors with them. Their blood sugar should ideally less than 200 mg/dL. Diabetic patients should monitor their glucose level and adjust their morning dose of insulin as needed for the prescribed meal.
- Additionally, it may be best to schedule exams for premenopausal women on days 1 to 10 of their menstrual cycle, to avoid the effects of hormonal changes on gastric emptying that has been shown in some, but not all studies.[6]
Technique or Treatment
To prepare the solid meal, the liquid egg whites are poured into a bowl, mixed with 0.5 to 1 mCi 99Tc sulfur colloid and cooked in a nonstick frying pan or microwave (Of note, simply adding the sulfur colloid after cooking the egg whites will result in poor labeling and lead to spurious measurements). The egg and radiopharmaceutical mixture should be stirred once or twice during cooking and cooked until it reaches the consistency of an omelet. The bread is toasted, jelly is spread on the toast, and a sandwich is made of the jellied bread and cooked egg mixture.[12] The meal should be consumed within 10 minutes, and imaging commences.
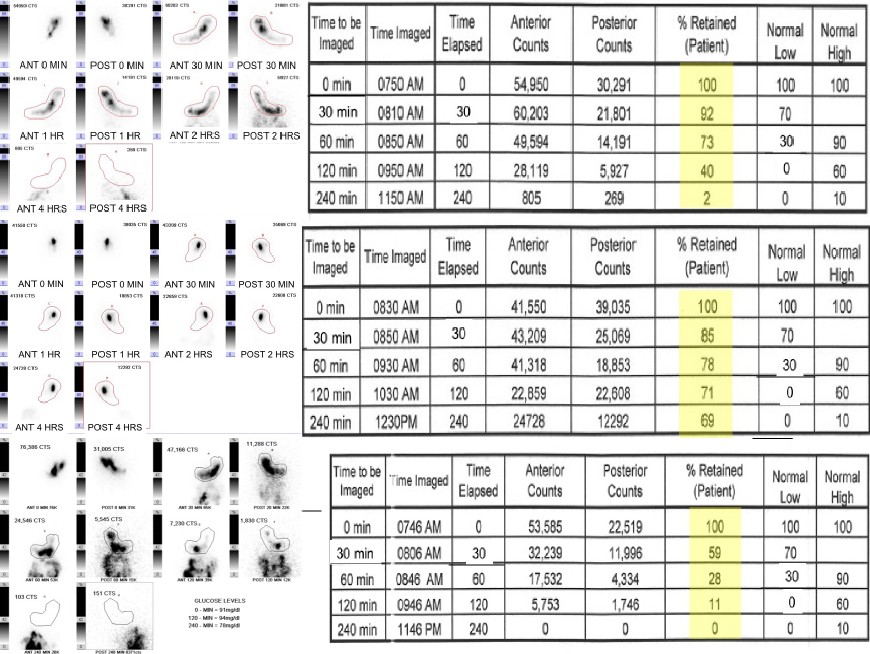
In addition to standardizing the meal, the consensus guidelines released in 2008 standardized the imaging and interpretation, endorsing a protocol developed in 2000 by Tougas and colleagues.[13] This simplified methodology for solid GES requires 1-minute images be acquired at only 4 time points: immediately after meal ingestion and at 1, 2, and 4 hours with an optional fifth time point at 30-minutes which can be helpful in the assessment of rapid gastric emptying.
The images are ideally acquired simultaneously in the anterior and posterior projections using a dual-head gamma camera with field-of-view (FOV) encompassing the entire stomach as well as the distal esophagus and proximal small bowel. If a dual-head gamma camera is not available, sequential anterior and then posterior images from a single-head gamma camera is an acceptable technique. The counts in the stomach are then measured by drawing a region-of-interest (ROI) around the stomach. Using the first time point (T=0) as the baseline (which includes all activity, to include any which has already traversed the stomach), the amount of activity retained in the stomach at each subsequent time point can be calculated using the geometric mean with decay correction and compared to validated normal values.
The published normal values are (FIG1)[14]:
- Thirty minutes: Greater than or equal to 70% meal retention
- One hour: 30% to 90% meal retention
- Two hours: Less than or equal to 60% meal retention
- Four hours: Less than or equal to 10% meal retention
- A retained meal value greater than 60% at 2 hours or 10% at 4 hours supports delayed gastric emptying (FIG2)
- A retained meal value less than 70% at 30 minutes or less than 30% at 1 hour suggests rapid gastric emptying (FIG3)[13][12]
If utilizing a liquid meal, the radiopharmaceutical is simply mixed with the 300 mL of water. The exam is then performed with the patient positioned semi-upright (45-degree angle) and imaging performed with a single-head gamma camera in the left anterior oblique projection (along the long axis of the stomach). Imaging starts immediately after the ingestion of the radiolabeled water with images acquired as 1-min frames continuously for 30 minutes. Like with solid gastric emptying, an ROI is drawn over the stomach to measure gastric retention. Unlike with solid gastric emptying, no geometric mean is calculated given the single-head camera technique and the rapid nature of liquid only emptying necessitates a time-activity-curve (TAC) be generated using each time point to calculate the time to reach 50% emptying (T-1/2). Using this protocol, a T-1/2 of fewer than 22 minutes is considered normal (mean plus 3 standard deviations).[10]
Complications
Factors that may affect the performance and negatively influence the clinical validity of a GES are:
- Incomplete meal consumption
- Slow meal consumption (taking longer than 10 minutes)
- Vomiting a portion of the meal
- Poor glycemic control
If these problems occur, they should be included in the exam report as well as a comment as to their potential impact on the accuracy of the results.
Clinical Significance
Gastroparesis and rapid gastric emptying are conditions of abnormal gastric motility in the absence of obstructive pathology.
Gastroparesis was classically thought to be the sequela of previous stomach surgery or the result of long-standing diabetes.[15] More recently, it has been found to most likely be idiopathic (32% of cases) with diabetes the second most common cause (29%) and surgery third (13%). Interestingly, women are afflicted 4-to-1 in comparison to men.[16] Given its most common presenting symptoms (nausea, pain, bloating) overlap with a multitude of other diseases, exact prevalence of delayed gastric emptying (DGE) is unknown, though it is estimated in the US that two-thirds of the country's 23 million people with diabetes suffer from gastroparesis while gastric dysmotility is present in 40% of adults with dyspepsia.[17]
Similar to DGE, rapid gastric empty is identified more commonly than previously suspected. It has been found in nearly 60% of patients with cyclic vomiting syndrome who undergo GES as well as a large proportion of individuals with autonomic dysfunction.[18]
Given this high prevalence of disease and its substantial impact on public health, it is critical that those afflicted be appropriately diagnosed to guide proper treatment and effective management. Key to this is the use of properly performed gastric emptying scintigraphy following standardized consensus guidelines. Research has shown that by using these parameters appropriately, the diagnostic yield of the GES can be improved significantly. Imaging of solid gastric emptying out to 4 hours, as recommended, increases sensitivity by a third over historical protocols that limited imaging to 2 or fewer hours. Adding a liquid GES study in patients with a normal solid GE can further increase detection of gastroparesis by another third.[19] By identifying more patients with abnormal gastric emptying, it will lead to more accurate diagnoses, which in turn will hopefully result in further development of therapies and improved care.
Enhancing Healthcare Team Outcomes
Disorders of gastric emptying often present with the symptoms of dyspepsia, post-prandial pain, bloating, early satiety, nausea, and vomiting. Such non-specific symptoms are frequently encountered by primary care providers, emergency department providers, surgeons, and gastroenterologists. The list of possible etiologies is extensive and includes gastric disorders, biliary disease, intestinal diseases, metabolic disorders, vascular pathology, and psychiatric diagnoses. Even after a thorough clinical history, physical exam and laboratory assessment, the definitive cause often remains in doubt. As such, subspecialty referrals are often sought, leading to these patients being evaluated by surgeons suspecting chronic cholecystitis, gastroenterologists concerned for peptic ulcer disease, vascular surgeons suspecting mesenteric ischemia, and mental health providers assessing for depression or anxiety. Of benefit to all these primary and subspecialty providers and their challenging patients is the well performed gastric emptying scintigraphy (GES) study following consensus guidelines. It provides a validated and reproducible means to accurately identify patients with gastroparesis or rapid gastric emptying as a potential source of their clinical complaints.