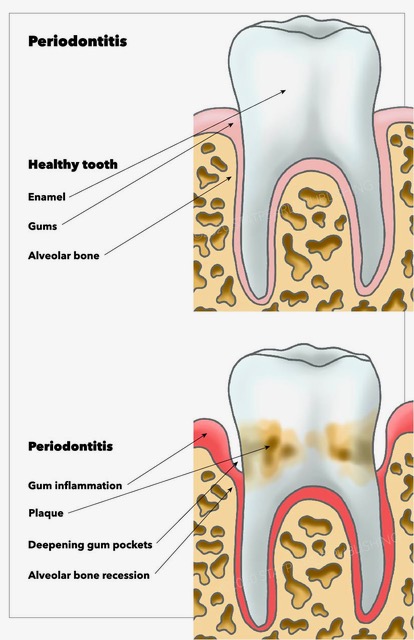
[1]
Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE. Defining the normal bacterial flora of the oral cavity. Journal of clinical microbiology. 2005 Nov:43(11):5721-32
[PubMed PMID: 16272510]
[2]
Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nature reviews. Microbiology. 2012 Oct:10(10):717-25. doi: 10.1038/nrmicro2873. Epub 2012 Sep 3
[PubMed PMID: 22941505]
[3]
Petersen PE, Baehni PC. Periodontal health and global public health. Periodontology 2000. 2012 Oct:60(1):7-14. doi: 10.1111/j.1600-0757.2012.00452.x. Epub
[PubMed PMID: 22909103]
[4]
Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000. 2013 Jun:62(1):203-17. doi: 10.1111/j.1600-0757.2012.00450.x. Epub
[PubMed PMID: 23574467]
[5]
Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr. Microbial complexes in subgingival plaque. Journal of clinical periodontology. 1998 Feb:25(2):134-44
[PubMed PMID: 9495612]
[6]
Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nature reviews. Microbiology. 2010 Jul:8(7):481-90. doi: 10.1038/nrmicro2337. Epub
[PubMed PMID: 20514045]
[7]
Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006 Sep:94(1):10-21
[PubMed PMID: 16998613]
[8]
Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends in molecular medicine. 2015 Mar:21(3):172-83. doi: 10.1016/j.molmed.2014.11.004. Epub 2014 Nov 20
[PubMed PMID: 25498392]
[9]
Shi B, Chang M, Martin J, Mitreva M, Lux R, Klokkevold P, Sodergren E, Weinstock GM, Haake SK, Li H. Dynamic changes in the subgingival microbiome and their potential for diagnosis and prognosis of periodontitis. mBio. 2015 Feb 17:6(1):e01926-14. doi: 10.1128/mBio.01926-14. Epub 2015 Feb 17
[PubMed PMID: 25691586]
[11]
LOE H, THEILADE E, JENSEN SB. EXPERIMENTAL GINGIVITIS IN MAN. The Journal of periodontology. 1965 May-Jun:36():177-87
[PubMed PMID: 14296927]
[12]
Theilade E, Wright WH, Jensen SB, Löe H. Experimental gingivitis in man. II. A longitudinal clinical and bacteriological investigation. Journal of periodontal research. 1966:1():1-13
[PubMed PMID: 4224181]
[13]
Armitage GC, Cullinan MP. Comparison of the clinical features of chronic and aggressive periodontitis. Periodontology 2000. 2010 Jun:53():12-27. doi: 10.1111/j.1600-0757.2010.00353.x. Epub
[PubMed PMID: 20403102]
[14]
Albandar JM. Aggressive periodontitis: case definition and diagnostic criteria. Periodontology 2000. 2014 Jun:65(1):13-26. doi: 10.1111/prd.12014. Epub
[PubMed PMID: 24738584]
Level 3 (low-level) evidence
[15]
Susin C, Haas AN, Albandar JM. Epidemiology and demographics of aggressive periodontitis. Periodontology 2000. 2014 Jun:65(1):27-45. doi: 10.1111/prd.12019. Epub
[PubMed PMID: 24738585]
[16]
Ababneh KT, Abu Hwaij ZM, Khader YS. Prevalence and risk indicators of gingivitis and periodontitis in a multi-centre study in North Jordan: a cross sectional study. BMC oral health. 2012 Jan 3:12():1. doi: 10.1186/1472-6831-12-1. Epub 2012 Jan 3
[PubMed PMID: 22214223]
[17]
Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in the United States, 1988-1994. Journal of periodontology. 1999 Jan:70(1):13-29
[PubMed PMID: 10052767]
[18]
Hillman JD, Socransky SS, Shivers M. The relationships between streptococcal species and periodontopathic bacteria in human dental plaque. Archives of oral biology. 1985:30(11-12):791-5
[PubMed PMID: 3868968]
[19]
Plančak D, Musić L, Puhar I. Quorum Sensing of Periodontal Pathogens. Acta stomatologica Croatica. 2015 Sep:49(3):234-41. doi: 10.15644/asc49/3/6. Epub
[PubMed PMID: 27688408]
[20]
Marsh PD. Dental plaque as a biofilm and a microbial community - implications for health and disease. BMC oral health. 2006 Jun 15:6 Suppl 1(Suppl 1):S14
[PubMed PMID: 16934115]
[22]
Jenkinson HF, Lamont RJ. Oral microbial communities in sickness and in health. Trends in microbiology. 2005 Dec:13(12):589-95
[PubMed PMID: 16214341]
[23]
SOCRANSKY SS, GIBBONS RJ, DALE AC, BORTNICK L, ROSENTHAL E, MACDONALD JB. The microbiota of the gingival crevice area of man. I. Total microscopic and viable counts and counts of specific organisms. Archives of oral biology. 1963 May-Jun:8():275-80
[PubMed PMID: 13989807]
[24]
Silva N, Abusleme L, Bravo D, Dutzan N, Garcia-Sesnich J, Vernal R, Hernández M, Gamonal J. Host response mechanisms in periodontal diseases. Journal of applied oral science : revista FOB. 2015 May-Jun:23(3):329-55. doi: 10.1590/1678-775720140259. Epub
[PubMed PMID: 26221929]
[25]
Hajishengallis G, Lambris JD. Microbial manipulation of receptor crosstalk in innate immunity. Nature reviews. Immunology. 2011 Mar:11(3):187-200. doi: 10.1038/nri2918. Epub
[PubMed PMID: 21350579]
[26]
Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Laboratory investigation; a journal of technical methods and pathology. 1976 Mar:34(3):235-49
[PubMed PMID: 765622]
[27]
Mitsis FJ. Hippocrates in the golden age: his life, his work and his contributions to dentistry. The Journal of the American College of Dentists. 1991 Spring:58(1):26-30
[PubMed PMID: 2066507]
[29]
Loesche WJ, Grossman NS. Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clinical microbiology reviews. 2001 Oct:14(4):727-52, table of contents
[PubMed PMID: 11585783]
[30]
Socransky SS, Haffajee AD, Smith C, Dibart S. Relation of counts of microbial species to clinical status at the sampled site. Journal of clinical periodontology. 1991 Nov:18(10):766-75
[PubMed PMID: 1661305]
[31]
Baer PN. The case for periodontosis as a clinical entity. Journal of periodontology. 1971 Aug:42(8):516-20
[PubMed PMID: 5284178]
Level 3 (low-level) evidence
[32]
Preshaw PM. Detection and diagnosis of periodontal conditions amenable to prevention. BMC oral health. 2015:15 Suppl 1(Suppl 1):S5. doi: 10.1186/1472-6831-15-S1-S5. Epub 2015 Sep 15
[PubMed PMID: 26390822]
[33]
Carrouel F, Viennot S, Santamaria J, Veber P, Bourgeois D. Quantitative Molecular Detection of 19 Major Pathogens in the Interdental Biofilm of Periodontally Healthy Young Adults. Frontiers in microbiology. 2016:7():840. doi: 10.3389/fmicb.2016.00840. Epub 2016 Jun 2
[PubMed PMID: 27313576]
[34]
Research, Science and Therapy Committee of the American Academy of Periodontology. Treatment of plaque-induced gingivitis, chronic periodontitis, and other clinical conditions. Journal of periodontology. 2001 Dec:72(12):1790-800
[PubMed PMID: 11811516]
[35]
Preshaw PM, Heasman L, Stacey F, Steen N, McCracken GI, Heasman PA. The effect of quitting smoking on chronic periodontitis. Journal of clinical periodontology. 2005 Aug:32(8):869-79
[PubMed PMID: 15998271]
[36]
Walker CB. The acquisition of antibiotic resistance in the periodontal microflora. Periodontology 2000. 1996 Feb:10():79-88
[PubMed PMID: 9567938]
[37]
Ramachandra SS, Gupta VV, Mehta DS, Gundavarapu KC, Luigi N. Differential Diagnosis between Chronic versus Aggressive Periodontitis and Staging of Aggressive Periodontitis: A Cross-sectional Study. Contemporary clinical dentistry. 2017 Oct-Dec:8(4):594-603. doi: 10.4103/ccd.ccd_623_17. Epub
[PubMed PMID: 29326511]
Level 2 (mid-level) evidence
[38]
McFall WT Jr. Tooth loss in 100 treated patients with periodontal disease. A long-term study. Journal of periodontology. 1982 Sep:53(9):539-49
[PubMed PMID: 6957591]
[39]
McGuire MK, Nunn ME. Prognosis versus actual outcome. II. The effectiveness of clinical parameters in developing an accurate prognosis. Journal of periodontology. 1996 Jul:67(7):658-65
[PubMed PMID: 8832476]
[40]
Lang NP, Joss A, Orsanic T, Gusberti FA, Siegrist BE. Bleeding on probing. A predictor for the progression of periodontal disease? Journal of clinical periodontology. 1986 Jul:13(6):590-6
[PubMed PMID: 3489010]
[41]
Page RC, Offenbacher S, Schroeder HE, Seymour GJ, Kornman KS. Advances in the pathogenesis of periodontitis: summary of developments, clinical implications and future directions. Periodontology 2000. 1997 Jun:14():216-48
[PubMed PMID: 9567973]
Level 3 (low-level) evidence
[42]
Beck JD, Slade G, Offenbacher S. Oral disease, cardiovascular disease and systemic inflammation. Periodontology 2000. 2000 Jun:23():110-20
[PubMed PMID: 11276757]
[43]
Axelsson P, Nyström B, Lindhe J. The long-term effect of a plaque control program on tooth mortality, caries and periodontal disease in adults. Results after 30 years of maintenance. Journal of clinical periodontology. 2004 Sep:31(9):749-57
[PubMed PMID: 15312097]
[44]
Axelsson P, Lindhe J. The significance of maintenance care in the treatment of periodontal disease. Journal of clinical periodontology. 1981 Aug:8(4):281-94
[PubMed PMID: 6947992]
[45]
Linden GJ, Herzberg MC, Working group 4 of joint EFP/AAP workshop. Periodontitis and systemic diseases: a record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of clinical periodontology. 2013 Apr:40 Suppl 14():S20-3. doi: 10.1111/jcpe.12091. Epub
[PubMed PMID: 23627330]
[46]
Heitz-Mayfield LJ, Trombelli L, Heitz F, Needleman I, Moles D. A systematic review of the effect of surgical debridement vs non-surgical debridement for the treatment of chronic periodontitis. Journal of clinical periodontology. 2002:29 Suppl 3():92-102; discussion 160-2
[PubMed PMID: 12787211]
Level 1 (high-level) evidence