Continuing Education Activity
A central line-associated bloodstream infection (CLABSI) is defined as the recovery of a pathogen from a blood culture in a patient who had a central line at the time of infection or within 48 hours before the development of infection. Most cases are preventable with proper aseptic techniques, surveillance, and management strategies. This activity describes the evaluation and management of patients undergoing central line placement and highlights the role of the interprofessional team in improving care for affected patients.
Objectives:
Describe the pathophysiology of CLABSI.
Review key history and physical exam findings of patients with CLABSI.
Summarize the treatment options for CLABSI.
Explain modalities to improve care coordination among interprofessional team members in order to improve outcomes for patients affected by CLABSI.
Introduction
A central line-associated bloodstream infection (CLABSI) is defined as the recovery of a pathogen from a blood culture in a patient who had a central line at the time of infection or within 48 hours before the development of infection. Of all the healthcare-associated infections, CLABSIs are associated with a high-cost burden, accounting for approximately $46,000 per case. Most cases are preventable with proper aseptic techniques, surveillance, and management strategies.[1][2]
Etiology
Based on the National Healthcare Safety Network (NHSN) data from January 2006 to October 2007, the order of selected pathogens associated with causing CLABSI is as follows. Gram-positive organisms (coagulase-negative staphylococci, 34.1%; enterococci, 16%; and Staphylococcus aureus, 9.9%) are the most common, followed by gram-negatives (Klebsiella, 5.8%; Enterobacter, 3.9%; Pseudomonas, 3.1%; E. coli, 2.7%; Acinetobacter, 2.2%), Candida species (11.8%), and others (10.5%).[3][4]
Tunneled hemodialysis catheters are prone to CRBSIs. Approximately 40%–80% of CRBSIs are caused by gram-positive organisms. Coagulase-negative Staphylococci, Staphylococcus aureus, and Enterococcus are the most common organisms. Methicillin-resistant staphylococcus is frequently seen. 20%–30% of infections CRBSIs are caused by gram-negative organisms[5].
Epidemiology
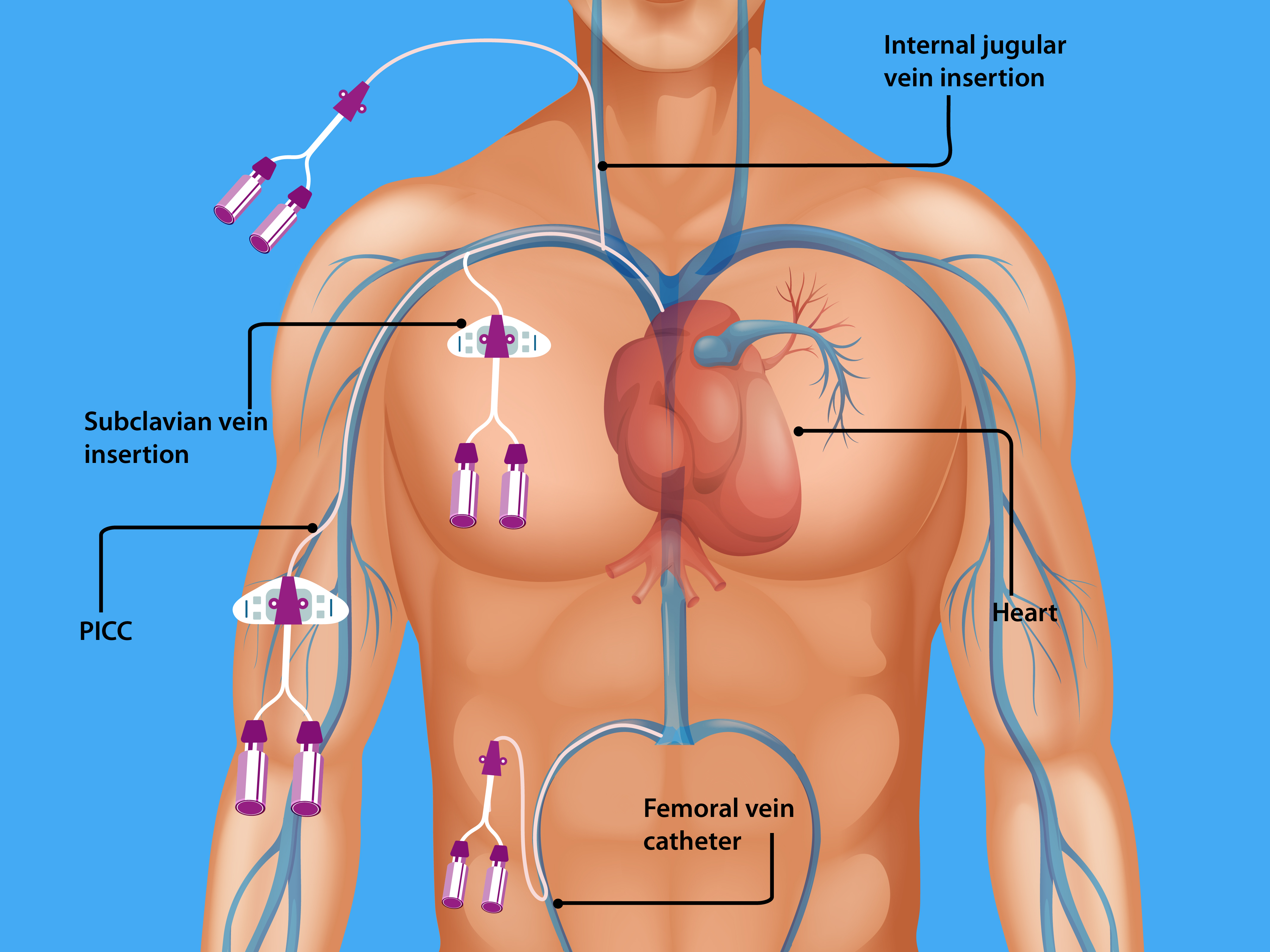
CLABSIs lead to prolonged hospital stays and increase healthcare costs and mortality. An estimated 250,000 bloodstream infections occur annually, and most are related to the presence of intravascular devices. In the United States, the CLABSI rate in intensive care units (ICU) is estimated to be 0.8 per 1000 central line days. International Nosocomial Infection Control Consortium (INICC) surveillance data from January 2010 through December 2015 (703 intensive care units in 50 countries) reported a CLABSI rate of 4.1 per 1000 central line days. Many central lines are found outside the ICUs. In one study, 55% of ICU and 24% of non-ICU patients had central lines. However, as more patients are located outside the ICU, 70% of hospitalized patients with central venous catheters were outside the ICU.[6]. In recent years, peripherally inserted central catheters (PICCs) have increased significantly, given some inherent advantages these devices offer [7]. Trained nursing staff can place PICC lines at the bedside with ultrasound guidance, allowing quick central venous access in both ICUs and general medical wards. Since PICCs are inserted in a peripheral vein of the arm with the tip advanced into a central vein (cavoatrial junction or the right atrium) by definition, they are central venous catheters. See Image. Central Venous Catheterization Body Access. The rates of CLABSI associated with PICCs are statistically similar to the conventional central venous catheters (CVCs) in the hospital setting[8]. In a multicenter study of 27,289 patients, CLABSI outcomes between the PICCs placed in the ICU compared to the general medical ward were similar. However, the study was limited, with an overall low number of events[9].
Pathophysiology
Central lines are of two types: (1) Tunneled catheters are implanted surgically (by creating a subcutaneous track before entering the vein) into the internal jugular, subclavian, or femoral vein for long-term (weeks to months) use such as chemotherapy or hemodialysis and (2) Non-tunneled catheters, more commonly used. They are temporary central venous catheters inserted percutaneously and account for most CLABSIs. Within 7 to 10 days of central venous catheter placement, bacteria on the skin surface migrate along the external surface of the catheter from the skin exit site towards the intravascular space. Typically, tunneled catheters have a cuff that causes a fibrotic reaction around the catheter, creating a barrier to bacterial migration). The absence of a tunnel (a subcutaneous tract) places non-tunneled catheters at higher risk for CLABSIs. CLABSIs that occur beyond ten days are usually caused by contamination of the hub (intraluminal), typically from a health care provider's contaminated hands but rarely from a host and often due to a breach of standard aseptic precautions to access the hub. Less common mechanisms include hematogenous seeding of bacteria from a contaminated infusate or another source.
Host factors that increase the risk of CLABSI are chronic illnesses (hemodialysis, malignancy, gastrointestinal tract disorders, pulmonary hypertension), immune-suppressed states (organ transplant, diabetes mellitus), malnutrition, total parenteral nutrition, extremes of age, loss of skin integrity (burns) and prolonged hospitalization before line insertion.
Femoral central venous catheters are associated with the highest risk of CLABSI, followed by internal jugular and subclavian catheters. Further, the catheter type, insertion conditions (emergent versus elective, use of full barrier precautions versus limited), catheter care, and operator skill also influence the risk of CLABSI.
Pseudomonas is commonly associated with neutropenia, severe illness, or known prior colonization. Candida is associated with risk factors: femoral catheterization, TPN, prolonged administration of broad-spectrum antibiotics, hematologic malignancy, or solid organ or hematopoietic stem cell transplantation. Certain bacteria such as staphylococci, Pseudomonas, and Candida produce extracellular polysaccharide [slime (biofilm)], which favors increased virulence, adherence to catheter surface, and resistance to antimicrobial therapy.[10]
History and Physical
Clinical manifestations vary depending on the severity of the illness. Fever and chills are the most common manifestations. Still, they may be masked if the patient is immunocompromised or at extremes of an age where atypical presentations of sepsis occur (altered mental status, hypotension, lethargy, fatigue). Exit site examination to look for signs of inflammation of tunneled catheters with inspection and palpation of the subcutaneous track is essential. Patients may report pain, swelling, or discharge from the exit site and redness surrounding or along the subcutaneous track when exit site or tunnel infections are present. For long-term catheters, difficulty drawing blood or poor flow are considered risk factors and manifestations of CLABSI.
Evaluation
In addition to the clinical exam, lab investigations are essential for diagnosis and management. Blood culture is the most crucial step towards diagnosis, in addition, to complete blood count, serum electrolytes, and renal and liver function tests, which are necessary to assess for severity and co-morbidities. In suspected cases, paired blood cultures (one from the central line and peripheral vein) must be drawn and labeled accordingly before being sent to the lab. In the case of poor peripheral access or when unable to obtain a peripheral sample, two or more samples must be drawn from different lumens of a multi-lumen central line. The following definitions help in arriving at a diagnosis:
Catheter-related bloodstream infection (CRBSI) - Infectious Diseases Society of America (IDSA) definition: Catheter-related bloodstream infection (CRBSI) is the preferred term used by IDSA. The definite diagnosis of CRBSI requires one of the following: Isolation of the same pathogen from a quantitative blood culture drawn through the central line and from a peripheral vein with the single bacterial colony count at least threefold higher in the sample from the central line as compared to that obtained from a peripheral vein (or) same organism recovered from percutaneous blood culture and quantitative (>15 colony-forming units) culture of the catheter tip (or) a shorter time to positive culture (>2 hours earlier) in the central line sample than the peripheral sample (differential time to positivity [ DTP ])[11]
CLABSI - Centers for Disease Control and Prevention (CDC) definition: CLABSI is a surveillance definition used by the CDC and defined as the recovery of a pathogen from a blood culture (a single blood culture for an organism not commonly present on the skin and two or more blood cultures for organism commonly present on the skin) in a patient who had a central line at the time of infection or within 48 hours before the development of infection. The infection cannot be related to any other infection the patient might have and must not have been present or incubating when the patient was admitted to the facility.[4]
In the case of tunneled catheters, the accepted definitions for exit site and tunnel infections are as follows:
Exit site infection: Signs of inflammation confined to an area (typically < 2 cm) surrounding the catheter exit site and the presence of exudate that proves to be culture-positive.
Tunnel infection: Inflammation extending beyond 2 cm from the exit site (along with the track or cephalad towards the vein entry site or extending beyond the cuff), typically associated with pain and tenderness along the subcutaneous track and culture-positive exudate at the exit site that may not be seen unless expressed by palpation.
Treatment / Management
When CLABSI is suspected, empiric therapy should be based on the most likely organism, host factors, and the overall clinical picture. While awaiting cultures, empiric treatment should be instituted promptly. In general, coverage for common gram-positive and gram-negative organisms is necessary. The local prevalence and antimicrobial susceptibility patterns in institutional antibiograms should be considered. The following are some considerations.
- Parenteral vancomycin if methicillin-resistant staphylococci (MRSA) is prevalent. Otherwise, parenteral anti-staphylococcal penicillin or cephalosporins such as nafcillin or cefazolin would suffice. If MRSA isolates, exhibit a minimum inhibitory concentration of > 2 mg/mL for vancomycin or, in the case of vancomycin-resistant enterococci (VRE), daptomycin is the drug of choice.
- The antibiotic choice for gram-negative coverage should be based on the risk of pseudomonal infection and local susceptibility patterns. If the risk of Pseudomonas is low, then a third-generation cephalosporin such as ceftriaxone is appropriate. In patients with a critical illness or high risk for resistant organisms, a combination of a beta-lactam (lactamase inhibitor) and an aminoglycoside is preferred - Cefepime or carbapenem with or without an aminoglycoside. Agents against Pseudomonas aeruginosa are required in the setting of neutropenia, severe illness, or known prior colonization.
- Echinocandins (micafungin, caspofungin, anidulafungin) are preferred agents for suspected candidemia if azole resistance is suspected (prior azole use or prevalent nonalbicans candida such as C.glabrata or C.krusei). Otherwise, intravenous fluconazole would suffice. Antifungal therapy must be considered in femoral catheterization, TPN, prolonged administration of broad-spectrum antibiotics, hematologic malignancy, or solid organ or bone marrow transplant recipients.
Once antimicrobial susceptibility results are available, de-escalation to specific and appropriate therapy is recommended. If blood cultures have no growth, the need for further empiric antibiotic treatment should be reassessed. Suppose unexplained fever or sepsis persisted in a patient with a short-term central venous or arterial catheter and paired peripheral venipuncture and catheter blood cultures have failed to identify CLABSI. In that case, the catheter should be removed, and the tip should be sent for culture.
All non-tunneled catheters causing CLABSI should be removed promptly, sometimes even before it is proven with the above criteria when clinical suspicion is high. Only for long-term catheters, salvage (systemic therapy coupled with antimicrobial lock [heparin + high concentration of antimicrobial agent that is selected based on susceptibility results]) can be attempted in limited instances such as:
- Uncomplicated CLABSI is caused by organisms other than Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus spp, Micrococcus species, Propionibacteria, fungi, or mycobacteria.
- The patients with limited vascular access sites or the ones who are solely dependent on central access for survival.
In the case of tunneled hemodialysis catheters, uncomplicated exit site infections can be treated with short-course topical or systemic antimicrobials. All tunnel infections will require catheter removal due to a high risk of CLABSI. Additional indications for removal of CLABSI from hemodialysis access include:
- Persistent symptoms > 36 hours or severe sepsis, hemodynamic instability(shock), and metastatic infection.
- Blood cultures that remain positive > 72 hours of appropriate antimicrobial therapy.
- Difficult to clear organisms (S. aureus, Pseudomonas, or fungi).
- Recurrence of uncomplicated CLABSI (any non-virulent organism) when salvaged for difficult access.
Duration of therapy: For patients with uncomplicated CRBSI (e.g., no endocarditis, or metastatic infection), in the absence of risk factors for hematogenous spread (e.g., hardware, immunosuppression) and negative blood cultures 72 hours of catheter removal, following approach for intravenous antimicrobial therapy is recommended:
- S. aureus - 14 days in the absence of endocarditis
- Coagulase-negative staphylococci - 7 days
- Enterococci and gram-negative bacilli - 10 to 14 days
- Candida - 14 days in the absence of retinitis
Differential Diagnosis
Pearls and Other Issues
Prevention Guidelines During Insertion
Recent data reveal no difference in the infection rate based on the insertion catheter site. The following are some key components of a prevention program, abstracted from an extensive list provided by the CDC and IDSA.[12][13][10]
- Hand hygiene by washing hands with soap, water, or alcohol-based gels or foams. Gloves do not prevent the need for hand hygiene.
- A strict aseptic technique using maximal sterile barrier precautions, including a full-body drape when inserting central venous catheters.
- Use 2% chlorhexidine skin preparations for disinfecting/ cleaning skin before insertion.
- Ultrasound guidance by an experienced provider for placement to circumvent mechanical complications and reduce the number of attempts.
- Avoid the femoral vein as a choice for central line placement, and prefer the subclavian vein when possible for non-tunneled catheters.
- Promptly remove any central line that is no longer required.
- Replace central lines placed during an emergency (asepsis not assured) as soon as possible or within 48 hours.
- Use a checklist.
Prevention Guidelines During Maintenance
- Disinfect the catheter hubs, injection ports, and connections before accessing the line.
- Replace administration sets other than sets used for lipids or blood products every 96 hours.
- Assess the need for the central line daily.
Enhancing Healthcare Team Outcomes
Central line-associated bloodstream infection (CLABSI) is a highly prevalent problem in the intensive care unit. These infections are associated with over 28,000 deaths yearly and cost over $2 billion. Only through best practices, protocols, checklists, and establishing a culture of patient safety in healthcare institutions can one reduce CLABSI to zero.[14] One of the significant reasons for central line removal is an infection or suspicion. This clinical practice leads to prolonged hospital stays and increased procedures and complication rates.
One of the challenges with central lines is the variety of catheter types inserted by diverse staff. In many hospitals, central lines are inserted by specialists, including anesthesiologists, surgeons, emergency room physicians, radiologists, and critical care physicians.[15]. PICC lines are often inserted by trained nursing staff. This heterogeneity has resulted in varied outcomes, but infections continue to be a common problem in almost every study.
Evidence-Based Medicine
Over the years, many guidelines have been established; some hospitals have a policy that for long-term access, the line can only be inserted by a dedicated team that consists of the surgeon, nurses, and a pharmacist who will monitor the patient. In addition, when administering TPN, one port is dedicated to nutrition. Plus, in some units, only nurses with training in central lines are allowed to infuse medications and other solutions. Evidence-based guidelines show that adhering to protocols can reduce the rate of CLABSI. However, audits of doctors who insert the central lines and nurses who monitor the lines for infections are vital to ensure compliance.[10] [Level 3]