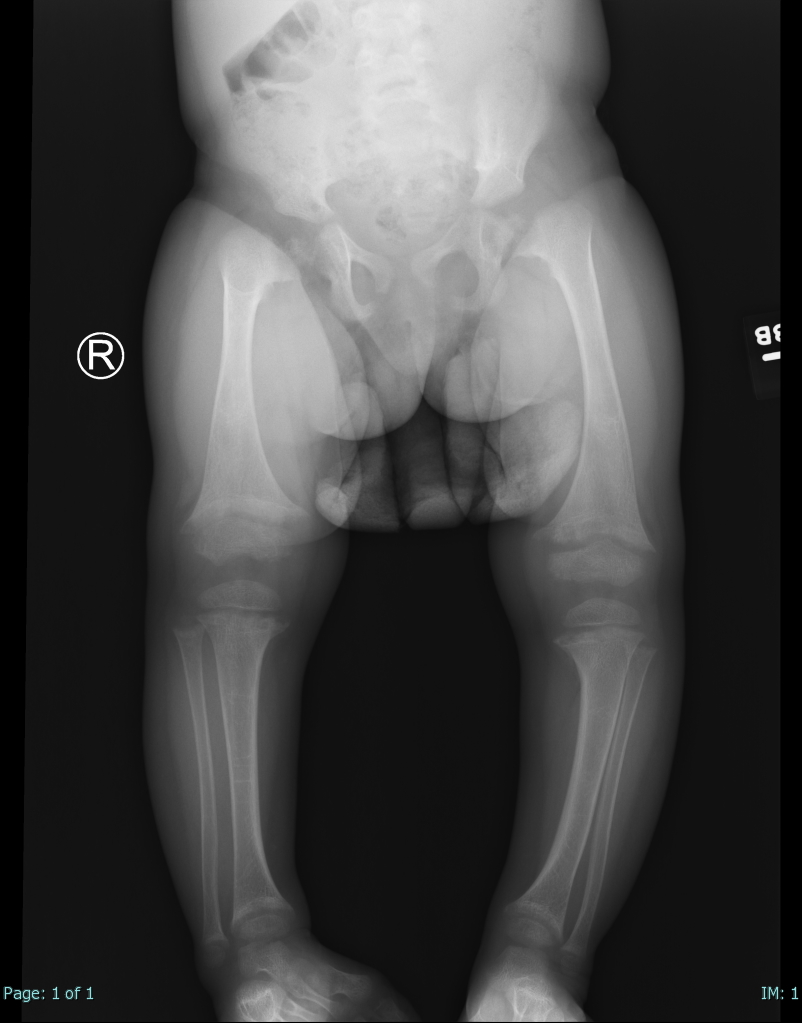
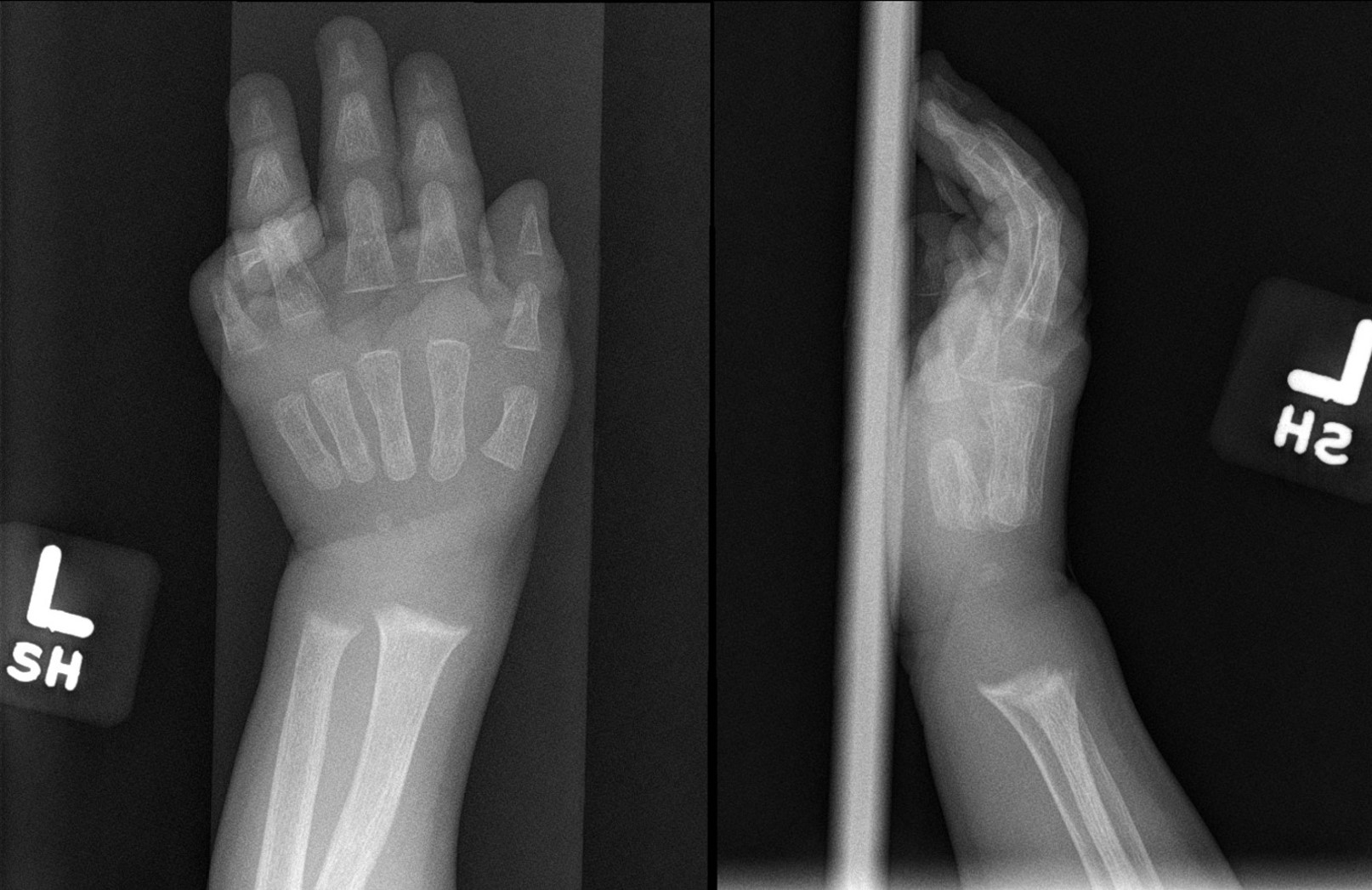
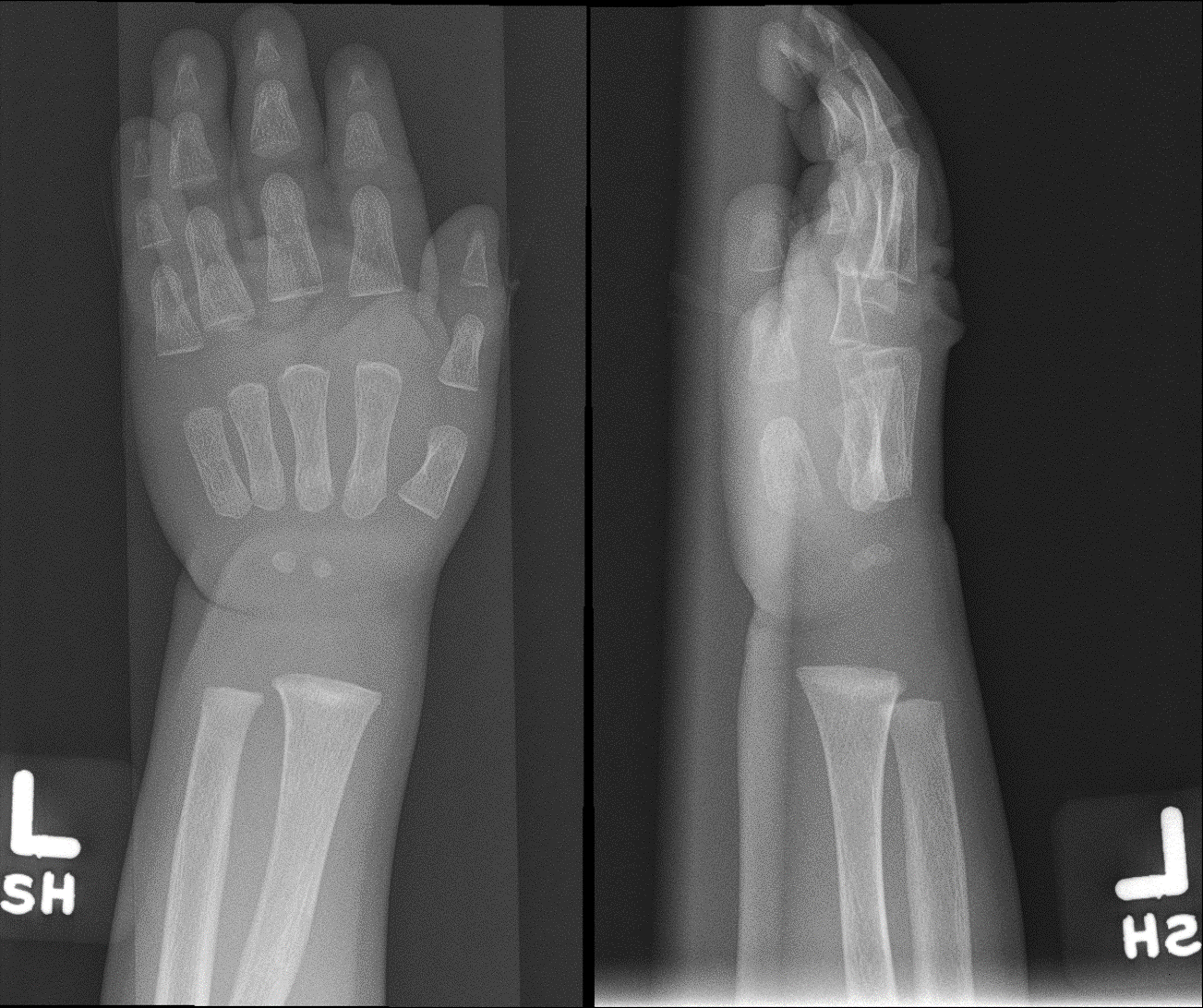
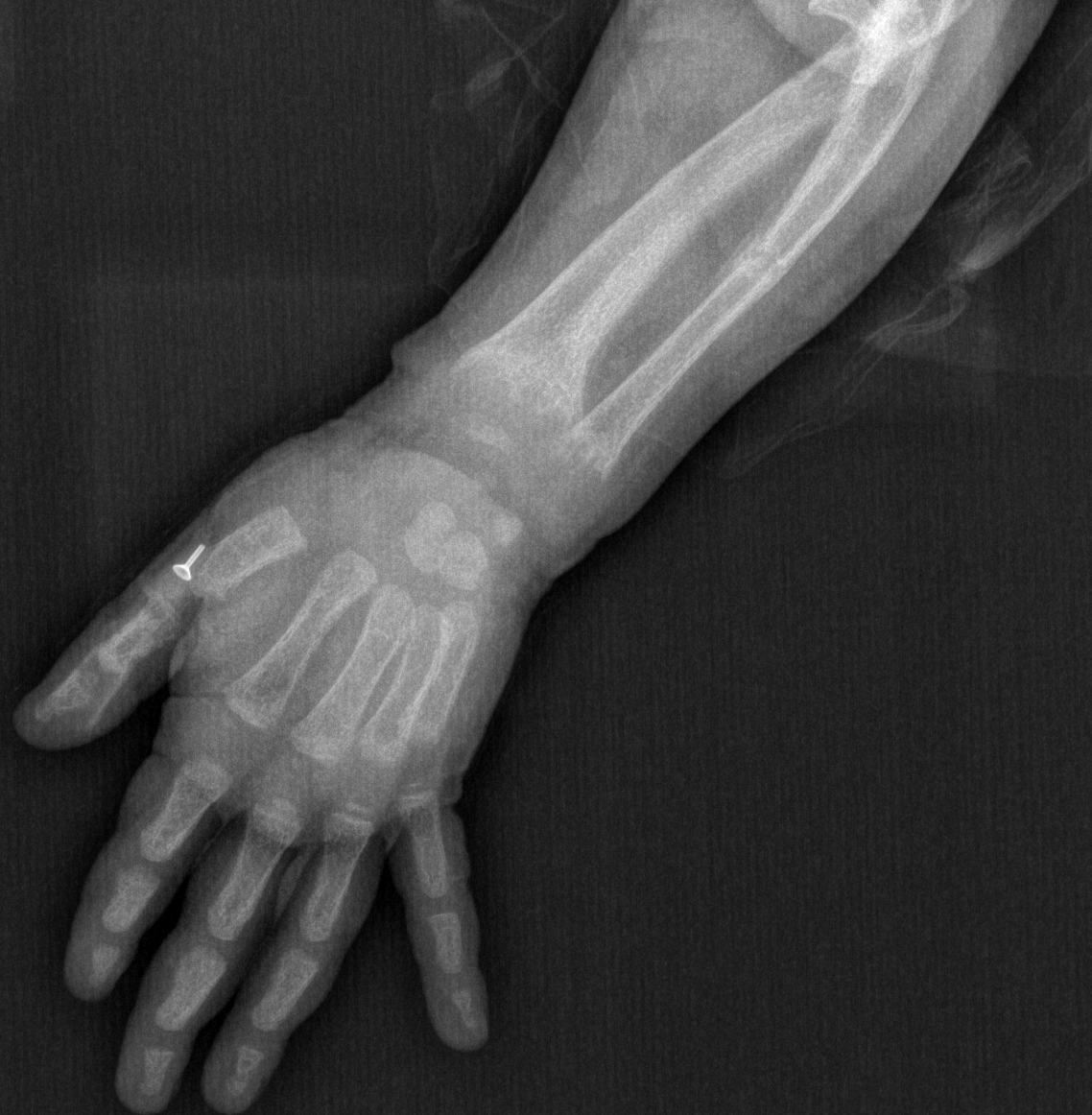
[1]
Pitt MJ. Rickets and osteomalacia are still around. Radiologic clinics of North America. 1991 Jan:29(1):97-118
[PubMed PMID: 1985332]
[3]
Shaw NJ. Prevention and treatment of nutritional rickets. The Journal of steroid biochemistry and molecular biology. 2016 Nov:164():145-147. doi: 10.1016/j.jsbmb.2015.10.014. Epub 2015 Oct 19
[PubMed PMID: 26493853]
[4]
Wheeler BJ, Snoddy AME, Munns C, Simm P, Siafarikas A, Jefferies C. A Brief History of Nutritional Rickets. Frontiers in endocrinology. 2019:10():795. doi: 10.3389/fendo.2019.00795. Epub 2019 Nov 14
[PubMed PMID: 31798536]
[5]
Fukumoto S, Ozono K, Michigami T, Minagawa M, Okazaki R, Sugimoto T, Takeuchi Y, Matsumoto T. Pathogenesis and diagnostic criteria for rickets and osteomalacia - proposal by an expert panel supported by Ministry of Health, Labour and Welfare, Japan, The Japanese Society for Bone and Mineral Research and The Japan Endocrine Society. Endocrine journal. 2015:62(8):665-71. doi: 10.1507/endocrj.EJ15-0289. Epub 2015 Jul 4
[PubMed PMID: 26156530]
[6]
Prentice A. Nutritional rickets around the world. The Journal of steroid biochemistry and molecular biology. 2013 Jul:136():201-6. doi: 10.1016/j.jsbmb.2012.11.018. Epub 2012 Dec 7
[PubMed PMID: 23220549]
[7]
Al-Sharafi BA, Al-Imad SA, Shamshair AM, Al-Faqeeh DH. Severe rickets in a young girl caused by celiac disease: the tragedy of delayed diagnosis: a case report. BMC research notes. 2014 Oct 8:7():701. doi: 10.1186/1756-0500-7-701. Epub 2014 Oct 8
[PubMed PMID: 25292220]
Level 3 (low-level) evidence
[8]
Mughal MZ. Rickets. Current osteoporosis reports. 2011 Dec:9(4):291-9. doi: 10.1007/s11914-011-0081-0. Epub
[PubMed PMID: 21968816]
[9]
Michałus I, Rusińska A. Rare, genetically conditioned forms of rickets: Differential diagnosis and advances in diagnostics and treatment. Clinical genetics. 2018 Jul:94(1):103-114. doi: 10.1111/cge.13229. Epub 2018 Mar 25
[PubMed PMID: 29417983]
Level 3 (low-level) evidence
[10]
Acar S, Demir K, Shi Y. Genetic Causes of Rickets. Journal of clinical research in pediatric endocrinology. 2017 Dec 30:9(Suppl 2):88-105. doi: 10.4274/jcrpe.2017.S008. Epub 2017 Dec 27
[PubMed PMID: 29280738]
[11]
Robinson PD, Högler W, Craig ME, Verge CF, Walker JL, Piper AC, Woodhead HJ, Cowell CT, Ambler GR. The re-emerging burden of rickets: a decade of experience from Sydney. Archives of disease in childhood. 2006 Jul:91(7):564-8
[PubMed PMID: 15956045]
[12]
Perez-Rossello JM, Feldman HA, Kleinman PK, Connolly SA, Fair RA, Myers RM, Gordon CM. Rachitic changes, demineralization, and fracture risk in healthy infants and toddlers with vitamin D deficiency. Radiology. 2012 Jan:262(1):234-41. doi: 10.1148/radiol.11110358. Epub 2011 Nov 21
[PubMed PMID: 22106354]
[13]
Dijkstra SH, van Beek A, Janssen JW, de Vleeschouwer LH, Huysman WA, van den Akker EL. High prevalence of vitamin D deficiency in newborn infants of high-risk mothers. Archives of disease in childhood. 2007 Sep:92(9):750-3
[PubMed PMID: 17715438]
[14]
Singleton R, Lescher R, Gessner BD, Benson M, Bulkow L, Rosenfeld J, Thomas T, Holman RC, Haberling D, Bruce M, Bartholomew M, Tiesinga J. Rickets and vitamin D deficiency in Alaska native children. Journal of pediatric endocrinology & metabolism : JPEM. 2015 Jul:28(7-8):815-23. doi: 10.1515/jpem-2014-0446. Epub
[PubMed PMID: 25741788]
[15]
Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet (London, England). 1982 Jan 9:1(8263):74-6
[PubMed PMID: 6119494]
[16]
Agarwal KS, Mughal MZ, Upadhyay P, Berry JL, Mawer EB, Puliyel JM. The impact of atmospheric pollution on vitamin D status of infants and toddlers in Delhi, India. Archives of disease in childhood. 2002 Aug:87(2):111-3
[PubMed PMID: 12138058]
[17]
Ozkan B. Nutritional rickets. Journal of clinical research in pediatric endocrinology. 2010:2(4):137-43. doi: 10.4274/jcrpe.v2i4.137. Epub 2010 Nov 1
[PubMed PMID: 21274312]
[18]
Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. Hormone research in paediatrics. 2016:85(2):83-106. doi: 10.1159/000443136. Epub 2016 Jan 8
[PubMed PMID: 26741135]
Level 3 (low-level) evidence
[19]
Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. The Journal of clinical endocrinology and metabolism. 2016 Feb:101(2):394-415. doi: 10.1210/jc.2015-2175. Epub 2016 Jan 8
[PubMed PMID: 26745253]
Level 3 (low-level) evidence
[20]
Ward LM, Gaboury I, Ladhani M, Zlotkin S. Vitamin D-deficiency rickets among children in Canada. CMAJ : Canadian Medical Association journal = journal de l'Association medicale canadienne. 2007 Jul 17:177(2):161-6
[PubMed PMID: 17600035]
[21]
Wheeler BJ, Dickson NP, Houghton LA, Ward LM, Taylor BJ. Incidence and characteristics of vitamin D deficiency rickets in New Zealand children: a New Zealand Paediatric Surveillance Unit study. Australian and New Zealand journal of public health. 2015 Aug:39(4):380-3. doi: 10.1111/1753-6405.12390. Epub 2015 Jun 29
[PubMed PMID: 26122859]
[22]
Munns CF, Simm PJ, Rodda CP, Garnett SP, Zacharin MR, Ward LM, Geddes J, Cherian S, Zurynski Y, Cowell CT, APSU Vitamin D Study Group. Incidence of vitamin D deficiency rickets among Australian children: an Australian Paediatric Surveillance Unit study. The Medical journal of Australia. 2012 Apr 16:196(7):466-8
[PubMed PMID: 22509879]
[23]
Callaghan AL, Moy RJ, Booth IW, Debelle G, Shaw NJ. Incidence of symptomatic vitamin D deficiency. Archives of disease in childhood. 2006 Jul:91(7):606-7
[PubMed PMID: 16595644]
[24]
Thacher TD, Fischer PR, Tebben PJ, Singh RJ, Cha SS, Maxson JA, Yawn BP. Increasing incidence of nutritional rickets: a population-based study in Olmsted County, Minnesota. Mayo Clinic proceedings. 2013 Feb:88(2):176-83. doi: 10.1016/j.mayocp.2012.10.018. Epub
[PubMed PMID: 23374621]
[25]
Goltzman D. Functions of vitamin D in bone. Histochemistry and cell biology. 2018 Apr:149(4):305-312. doi: 10.1007/s00418-018-1648-y. Epub 2018 Feb 12
[PubMed PMID: 29435763]
[26]
Nield LS, Mahajan P, Joshi A, Kamat D. Rickets: not a disease of the past. American family physician. 2006 Aug 15:74(4):619-26
[PubMed PMID: 16939184]
[27]
Hanafy MM, Hassanein ES, el Khateeb S. Benign intracranial hypertension in vitamin D deficiency rickets associated with malnutrition. The Journal of tropical pediatrics and African child health. 1967 Mar:13(1):19-22
[PubMed PMID: 5298825]
[28]
Vakharia JD, Matlock K, Taylor HO, Backeljauw PF, Topor LS. Craniosynostosis as the Presenting Feature of X-linked Hypophosphatemic Rickets. Pediatrics. 2018 Apr:141(Suppl 5):S515-S519. doi: 10.1542/peds.2017-2522. Epub
[PubMed PMID: 29610183]
[29]
Lambert AS, Linglart A. Hypocalcaemic and hypophosphatemic rickets. Best practice & research. Clinical endocrinology & metabolism. 2018 Aug:32(4):455-476. doi: 10.1016/j.beem.2018.05.009. Epub 2018 Jul 4
[PubMed PMID: 30086869]
[30]
Bailie GR, Johnson CA. Comparative review of the pharmacokinetics of vitamin D analogues. Seminars in dialysis. 2002 Sep-Oct:15(5):352-7
[PubMed PMID: 12358640]
Level 2 (mid-level) evidence
[32]
Chanchlani R, Nemer P, Sinha R, Nemer L, Krishnappa V, Sochett E, Safadi F, Raina R. An Overview of Rickets in Children. Kidney international reports. 2020 Jul:5(7):980-990. doi: 10.1016/j.ekir.2020.03.025. Epub 2020 Apr 11
[PubMed PMID: 32647755]
Level 3 (low-level) evidence
[33]
Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE. Treatment of hypovitaminosis D in infants and toddlers. The Journal of clinical endocrinology and metabolism. 2008 Jul:93(7):2716-21. doi: 10.1210/jc.2007-2790. Epub 2008 Apr 15
[PubMed PMID: 18413426]
[34]
Shah BR, Finberg L. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. The Journal of pediatrics. 1994 Sep:125(3):487-90
[PubMed PMID: 8071764]
[35]
Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM, Endocrine Society. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2011 Jul:96(7):1911-30. doi: 10.1210/jc.2011-0385. Epub 2011 Jun 6
[PubMed PMID: 21646368]
Level 1 (high-level) evidence
[36]
Hatun Ş, Ozkan B, Bereket A. Vitamin D deficiency and prevention: Turkish experience. Acta paediatrica (Oslo, Norway : 1992). 2011 Sep:100(9):1195-9. doi: 10.1111/j.1651-2227.2011.02383.x. Epub 2011 Jul 4
[PubMed PMID: 21672012]
[37]
Skalova S, Kutilek S. Transient hyperphosphatemia: a benign laboratory disorder in a boy with Gitelman syndrome. Jornal brasileiro de nefrologia. 2016 Jul-Sep:38(3):363-365. doi: 10.5935/0101-2800.20160055. Epub
[PubMed PMID: 27737396]