[1]
Kaufman J, Dhakal M, Patel B, Hamburger R. Community-acquired acute renal failure. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1991 Feb:17(2):191-8
[PubMed PMID: 1992662]
[2]
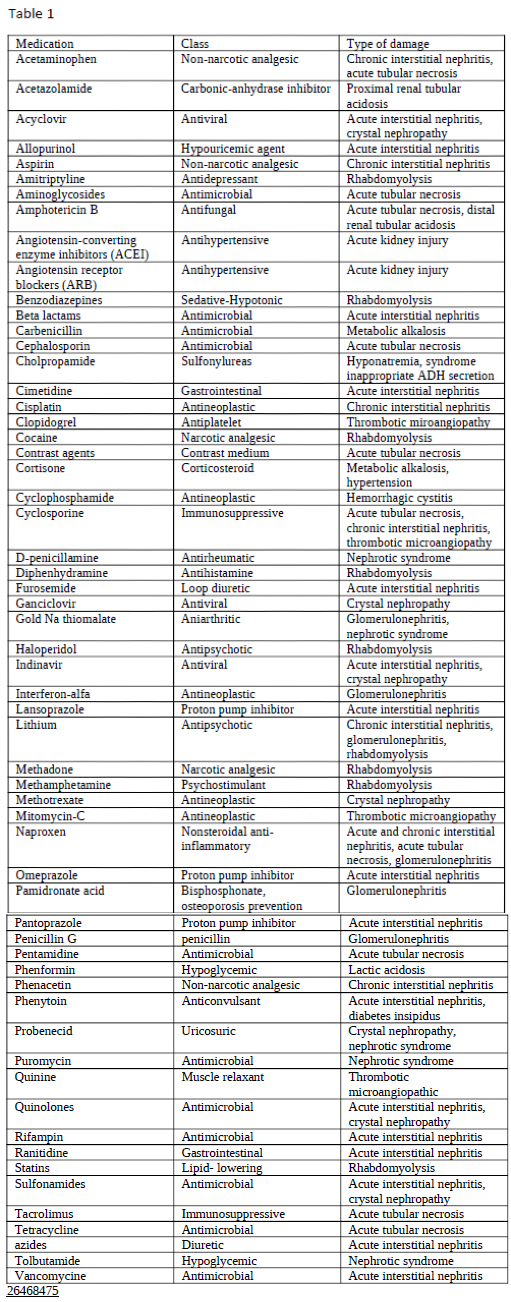
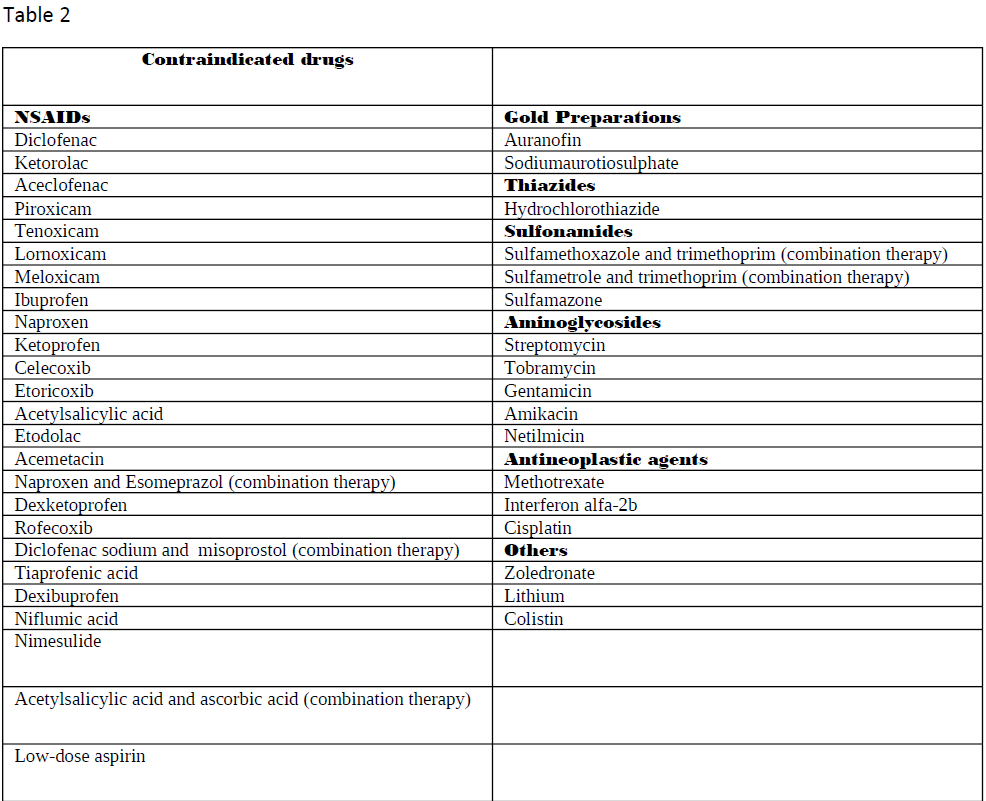
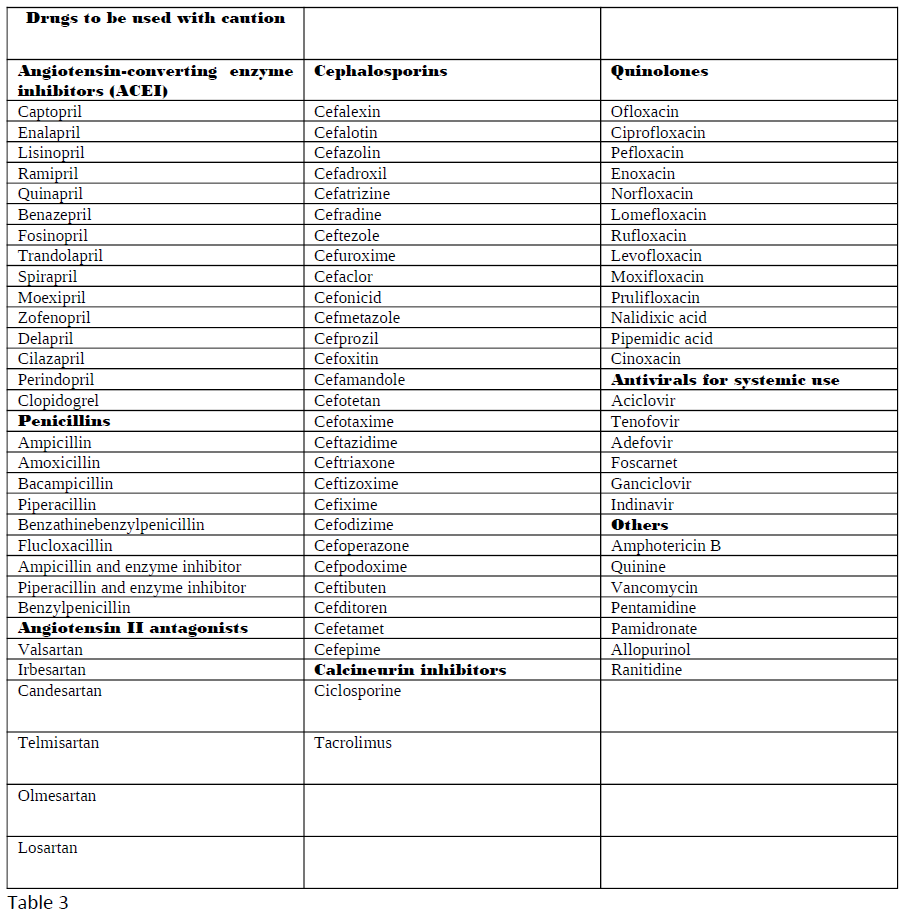
Ghane Shahrbaf F, Assadi F. Drug-induced renal disorders. Journal of renal injury prevention. 2015:4(3):57-60. doi: 10.12861/jrip.2015.12. Epub 2015 Sep 1
[PubMed PMID: 26468475]
[3]
Schetz M, Dasta J, Goldstein S, Golper T. Drug-induced acute kidney injury. Current opinion in critical care. 2005 Dec:11(6):555-65
[PubMed PMID: 16292059]
Level 3 (low-level) evidence
[4]
Perazella MA. Drug-induced nephropathy: an update. Expert opinion on drug safety. 2005 Jul:4(4):689-706
[PubMed PMID: 16011448]
Level 3 (low-level) evidence
[5]
Markowitz GS, Fine PL, Stack JI, Kunis CL, Radhakrishnan J, Palecki W, Park J, Nasr SH, Hoh S, Siegel DS, D'Agati VD. Toxic acute tubular necrosis following treatment with zoledronate (Zometa). Kidney international. 2003 Jul:64(1):281-9
[PubMed PMID: 12787420]
[6]
Perneger TV, Whelton PK, Klag MJ. Risk of kidney failure associated with the use of acetaminophen, aspirin, and nonsteroidal antiinflammatory drugs. The New England journal of medicine. 1994 Dec 22:331(25):1675-9
[PubMed PMID: 7969358]
[7]
Markowitz GS, Perazella MA. Drug-induced renal failure: a focus on tubulointerstitial disease. Clinica chimica acta; international journal of clinical chemistry. 2005 Jan:351(1-2):31-47
[PubMed PMID: 15563870]
[8]
Ingrasciotta Y, Sultana J, Giorgianni F, Caputi AP, Arcoraci V, Tari DU, Linguiti C, Perrotta M, Nucita A, Pellegrini F, Fontana A, Cavagna L, Santoro D, Trifirò G. The burden of nephrotoxic drug prescriptions in patients with chronic kidney disease: a retrospective population-based study in Southern Italy. PloS one. 2014:9(2):e89072. doi: 10.1371/journal.pone.0089072. Epub 2014 Feb 18
[PubMed PMID: 24558471]
Level 2 (mid-level) evidence
[9]
Ferguson MA, Vaidya VS, Bonventre JV. Biomarkers of nephrotoxic acute kidney injury. Toxicology. 2008 Mar 20:245(3):182-93. doi: 10.1016/j.tox.2007.12.024. Epub 2008 Jan 4
[PubMed PMID: 18294749]
[10]
Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annual review of pharmacology and toxicology. 2008:48():463-93
[PubMed PMID: 17937594]
[11]
Nerlich AG, Schleicher ED, Wiest I, Specks U, Timpl R. Immunohistochemical localization of collagen VI in diabetic glomeruli. Kidney international. 1994 Jun:45(6):1648-56
[PubMed PMID: 7933812]
[12]
Alchi B, Nishi S, Kondo D, Kaneko Y, Matsuki A, Imai N, Ueno M, Iguchi S, Sakatsume M, Narita I, Yamamoto T, Gejyo F. Osteopontin expression in acute renal allograft rejection. Kidney international. 2005 Mar:67(3):886-96
[PubMed PMID: 15698428]
[13]
Guo X, Nzerue C. How to prevent, recognize, and treat drug-induced nephrotoxicity. Cleveland Clinic journal of medicine. 2002 Apr:69(4):289-90, 293-4, 296-7 passim
[PubMed PMID: 11996200]
[14]
Kim SY, Moon A. Drug-induced nephrotoxicity and its biomarkers. Biomolecules & therapeutics. 2012 May:20(3):268-72. doi: 10.4062/biomolther.2012.20.3.268. Epub
[PubMed PMID: 24130922]
[15]
Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nature clinical practice. Nephrology. 2006 Feb:2(2):80-91
[PubMed PMID: 16932399]
[16]
Bonventre JV, Vaidya VS, Schmouder R, Feig P, Dieterle F. Next-generation biomarkers for detecting kidney toxicity. Nature biotechnology. 2010 May:28(5):436-40. doi: 10.1038/nbt0510-436. Epub
[PubMed PMID: 20458311]