Continuing Education Activity
Filoviruses can cause high morbidity and mortality due to severe disease that commonly involves vomiting, diarrhea, and infrequently hemorrhaging and a comatose state leading to death, also known as Ebola infection. This activity reviews the diagnosis and management of filovirus diseases with a focus on the Ebola virus disease and emphasizes the interprofessional and public health role in managing patients with this disease and containing outbreaks.
Objectives:
- Identify the epidemiology of filovirus.
- Describe the history and physical findings expected with filoviruses.
- Outline the treatment and management options available for filoviruses.
- Summarize interprofessional team strategies for improving care coordination and communication to treat and contain filovirus infections/outbreaks and to improve outcomes.
Introduction
Filoviruses, viral family Filoviridae, can cause hemorrhagic fever in humans and primates. There are three known genera of filoviruses: Cuevavirus, Marburgvirus, and Ebolavirus. These further subdivide into six species: Zaire, Sudan, Tai Forest, Bundibugyo, Reston, and Bombali. Thus far, the Reston and Bombali strains are not known to cause disease in humans.[1]
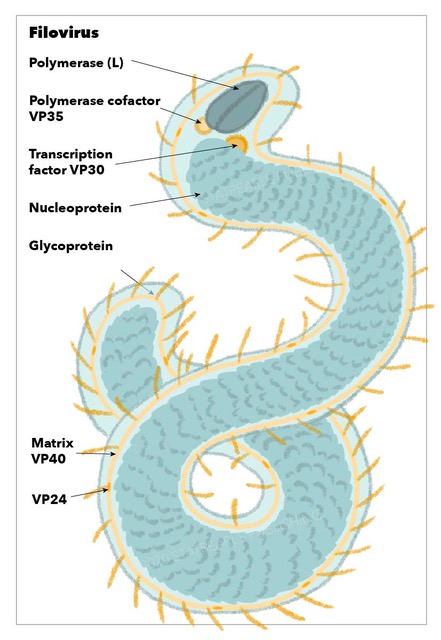
Structurally, filoviruses obtained their name due to their macroscopic appearance as filamentous viruses, from the Latin filum for "thread," often having a stringlike or torus-like appearance. They have enveloped particles that contain a negative-sense single-stranded RNA genome with genes arranged linearly. Additionally, they have a single glycoprotein spike on the surface and four structural proteins which include the virus-encoded polymerase.[1] Filoviruses are highly contagious and spread quickly via human-human contact. They are considered biosafety level 4 agents with very high mortality rates.[2]
Etiology
Ebola virus infects a broad range of cell types. After attachment to the host cell, mediated by the surface glycoprotein, the virus gets internalized via macropinocytosis. Binding efficacy of the virus is related to acid sphingomyelinase and plasma membrane sphingomyelin on the host cell.[3] On the viral side, attachment gets mediated via its a glycoprotein, a triplet of heterodimers each containing a binding and fusion subunit. Once internalized into the host cell, the virus travels into the late endosome where cysteine protease activates the fusogenic form of the surface glycoprotein which exposes the binding domain and results in fusion of the viral envelope with the endosome membrane releasing the viral nucleocapsid into the cytoplasm with resultant transcription, replication, and budding of new viruses. After infection begins, the virus spreads to many cell types with resultant generalized organ failure.[2]
Epidemiology
The first discovery of filovirus was in 1976 and since that time has had over twenty known outbreaks, mainly located in sub-Saharan Africa in isolated rural areas and focused around Sudan, Uganda, Democratic Republic of Congo, and Gabon. To date, the largest known outbreak occurred in West Africa from 2013 to 2016. This outbreak involved multiple countries, both rural and urban areas, and had a high mortality rate with over 28000 infections and 11000 deaths. However, this number may be optimistic as the actual number is thought to be much higher due to under-reporting. Thus far, the ebolavirus outbreaks in humans have been constrained to African countries, but this could change easily with international travel and secondary cases have been reported in Spain and the USA.
Ebolavirus is zoonotic with fruit bats of the Pteropodidae family currently thought to be the natural hosts of the disease. In natural conditions, scientists have yet to isolate the virus in bats. Transmission to humans likely occurs with handling sick/infected forest animals. Secondary spread via human-human transmission occurs via a direct contract of infected body fluids.
Pathophysiology
The virus enters the body through mucous membranes and infects cells such as macrophages and dendritic cells. The filovirus then replicates within causing their necrosis and subsequent release of thousands of new viral particles into the body. The virus then spreads systemically in a rapid fashion through suppression of Type-I interferon response. Systemic spread to lymph nodes, spleen, liver, and thymus and other immunologic tissue results in further virus propagation. Autopsies show that fatal disease usually results in necrosis of tissues of organs such as liver and spleen.[1] The body reacts to filovirus infection through a systemic inflammatory response releasing various cytokines, which can contribute to symptoms of shock, nausea, vomiting, vascular leak, coagulation defects, and volume depletion.[3]
History and Physical
A thorough patient history and physical exam are important in the diagnosis and evaluation of any disease, and for filoviruses, special care must be taken with regards to travel history, exposure history, and sick contacts. Obtaining the type, duration, and onset of symptoms is necessary. Generalized symptoms include prodromal fever, nausea, vomiting, diarrhea, muscle pain, weakness, fatigue, headache, abdominal pain, and unexplained hemorrhage. Symptoms generally appear 2 to 21 days after contact (average 8 to 10 days) and are commonly mistaken for flu or malaria. Hemorrhagic symptoms can vary and occur in approximately 30 to 50% of patients and often include mucosal bleeding, particularly involving the gastrointestinal and genitourinary tracts.[4]
Evaluation
In addition to the history and physical exam, general lab tests will typically demonstrate signs of intravascular volume depletion from gastrointestinal volume losses along with extensive liver and renal lab abnormalities. Indirect identification of viral particles, proteins or RNA from blood, serum or plasma is used for diagnosis of filovirus infection with RNA nucleic acid amplification testing being the preferred method. These tests confer a significant risk of lab-acquired infections and patient samples are an extreme biohazard risk and require special laboratory procedures. Definitive confirmation of infection is only by virus isolation. IgM testing can be diagnostic during the convalescent phase of illness and IgG is generally only monitored for epidemiologic surveillance. Rapid diagnostic tests were developed as a result of the 2016 Ebola outbreak with five tests approved for Ebola virus. These tests utilize the same antibody/antigen capture as an ELISA test but are done in a lateral flow strip allowing faster results with less processing. Rapid testing is critical in controlling outbreaks and preventing the spread of the virus.[1]
Treatment / Management
Managing filovirus disease typically focuses on prevention/detection and supportive care with other management strategies beginning to emerge. There are very few randomized controlled trials on Ebola treatment. With only one on specific anti-ebola therapy, ZMapp, which enrolled 72 patients and compared ZMapp, a monoclonal antibody, vs. standard care with resultant 22% vs. 37% mortality. Another international multisite retrospective cohort study has looked at intravenous fluid therapy compared to oral hydration and found no statistical difference in 28-day survival among the studied 424 participants.[5]
Studies have examined convalescent plasma with unclear results. Two studies revealed decreased mortality rates with convalescent plasma (31% vs. 38% and 28% vs. 44%), but these studies had limitations including lack of randomization, lack of data on other interventions, and an unknown level of neutralizing antibodies in plasma. Their small sample sizes also limited these studies.
A non-randomized single-arm study with 99 patients studied favipiravir Patients tolerated it well with a reduction in mortality of 44% vs. 65%; however, this data is limited by small sample sizes, non-randomization, and lack of control for between-group treatments.[6]
Interferon beta 1-a has been studied in a non-randomized single-arm study and found a reduction in 21-day mortality from 84% to 33%. Data limitations included small sample size and non-randomization.[6]
Also, palliative care has been suggested as an essential element for the treatment of these diseases given their high fatality rate, lack of effective treatment, and the crisis nature of outbreaks. Palliative services are critical to preventing and alleviating the suffering of patients in such a high fatality disease with significant additional psycho-social strain associated with outbreaks, infection, and symptom burden.[5]
Other molecular studies have identified agents not specific to Ebola virus that may aid in treatment. Ion channel inhibitors, such as amiodarone, have shown an affinity for preventing filovirus entry into cells in vitro, this inhibition is attainable at normal treatment doses for arrhythmia. Also, amiodarone interferes with glycoprotein processing with resultant inhibition of the fusion of the viral envelope and the endosomal membrane. However, these in vitro studies have not shown any clinical improvements in treatment during outbreaks. Antiparasitic drugs, such as chloroquine, has been shown to have antiviral properties by inhibiting viral entry and reducing inflammation. However, despite promising in vitro studies, human studies have not demonstrated the efficacy of antiparasitic drugs in the Ebola virus infection. Psychoactive drugs, such as chlorpromazine and first-generation anti-histamines, also act by inhibiting viral internalization. Selective estrogen receptor modulators also inhibit viral entry independent of estrogen receptors on target cells with the exact mechanism remaining unknown with two animal studies showing promise for protection from Ebola virus infection, one of which confirmed protection while the other obtained 50% protection rates.[7][3][8]
During the 2014 to 2016 outbreak, two vaccines underwent phase II/III trials and showed promise with high efficacy and long term antibody responses for Ebola virus. Marburgvirus, another filovirus, to this day does not have a vaccine.[9]
Differential Diagnosis
Ebola virus and marburgvirus disease must be considerations in patients who have traveled to areas where they are endemic or have had reported outbreaks.
Nonspecific viral prodrome and fever make specific diagnosis difficult, and filovirus infection can be easily mistaken for malaria, typhoid fever, influenza, Crimean-congo hemorrhagic fever, an acute surgical abdomen, cholera, and other hemorrhagic fevers such as dengue, Lassa and yellow fever.[9]
Toxicity and Adverse Effect Management
Treatment of filoviral infection is mainly supportive care with fluid and electrolyte replacement, respiratory support, and antimicrobial therapy. A few investigative therapies are currently under development.[10]
Prognosis
The prognosis for filovirus infection tends to be quite poor with few treatment options and high mortality rates that vary between 30% and 70% with prognosis worsening with increasing patient age and nutritional status.
Promisingly, vaccines have undergone phase II/III clinical trials during the 2014 to 2016 outbreak.[9]
Complications
In severe forms of the disease, death usually occurs around 6 to 16 days from symptom onset. The cause of death is typically shock and multiorgan failure, primarily hepatic and renal failure, also including thrombocytopenia and coagulopathy.[6]
Deterrence and Patient Education
Public health interventions should aim at containing the epidemic and attempt to minimize transmission both in the healthcare setting and the community. Additionally, to the coordinated local response, the WHO under the Global Outbreak Alert and Response network coordinate a global response. Outbreak containment has seen success in Uganda due to strong political support with the development of local task forces, public education, effective coordination, prompt utilization of community resources and efficient laboratory systems. Cases of confirmed or suspected filovirus infection should undergo management in isolated facilities within affected communities.[11]
Patients should receive education regarding the risks and high mortality associated with filovirus infection along with routes of spread, including bodily fluids and sexual transmission. Additionally, the importance of reporting suspected cases should have reinforcement, and home treatment should also be strongly discouraged.[12]
Enhancing Healthcare Team Outcomes
Management of filovirus disease and an outbreak is an interprofessional undertaking requiring the mobilization of not just resources within the hospital managing the patient, but also local resources to contain the spread of disease, identify infected individuals, and educate the population. National and global resources such as the Centers for Disease Control (CDC) and the World Health Organization (WHO) assist in managing the local cases, identify the origin of the outbreak, prevent spread, provide diagnostic aid from high-level biosafety laboratories, and provide experimental treatment options.[13]
Within the hospital, multiple specialists are needed to coordinate the care of critically ill patients requiring input from gastroenterology, nephrology, infectious disease, hematology, specialty-trained nursing, pharmacy, among possibly many others. Infectious disease board-certified pharmacists can assist in the supportive measures or cutting edge unproven therapies if desired. Nursing will provide supportive care and monitor the patient's condition, reporting any status changes to the team. Furthermore, coordination of containment within the hospital and minimization lab draws and therefore, of exposure risks for healthcare workers is essential given the possibility of laboratory-acquired infection and person-person transmission. Thus, an interprofessional team approach is needed for both care and containment. [6] [Level 5]