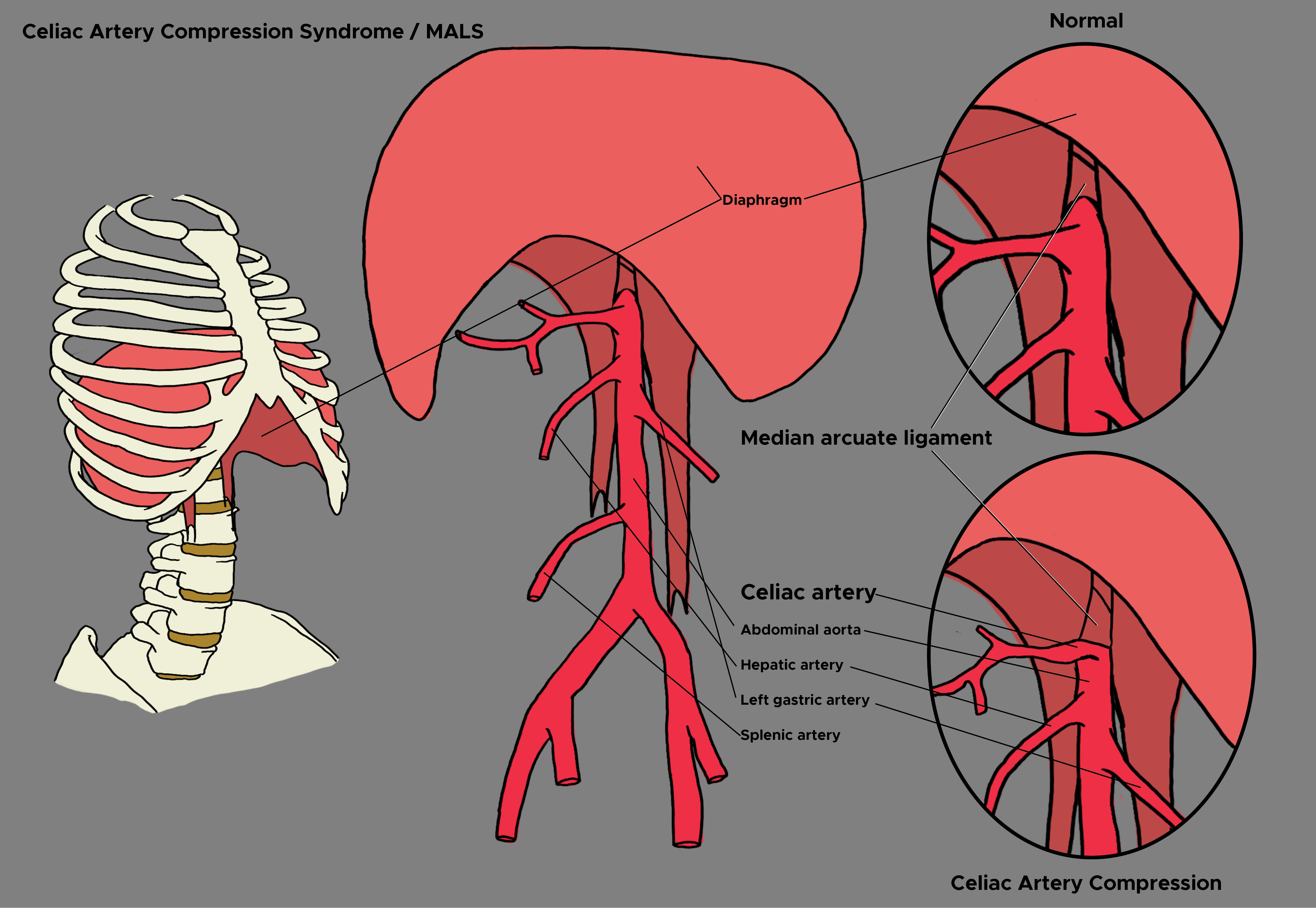
[1]
Zambrano-Lara M,Gonzalez-Urquijo M,Lozano-Balderas G,Rodarte-Shade M,Fabiani MA, Median arcuate ligament syndrome as a rare cause of chronic abdominal pain. Revista de gastroenterologia de Mexico. 2020 Jul 14;
[PubMed PMID: 32680594]
[2]
Matsuura H,Okita A,Suganami Y, Intermittent Severe Epigastric Pain and Abdominal Bruit Varying With Respiration. Gastroenterology. 2020 Mar;
[PubMed PMID: 31560895]
[3]
Koç M,Artaş H,Serhatlıoğlu S, The investigation of incidence and multidetector computed tomography findings of median arcuate ligament syndrome Turkish journal of medical sciences. 2018 Dec 12;
[PubMed PMID: 30541249]
[4]
Den B,Ignashov AM,Perleĭ VE,Gichkin AIu,Ustiuzhaninov AS, [The significance of respiratory and orthostatic tests in duplex scanning in diagnostics of celiac artery compression syndrome]. Vestnik khirurgii imeni I. I. Grekova. 2013;
[PubMed PMID: 24000675]
[5]
Römer C,Fischer T,Haase O,Möckel M,Hamm B,Lerchbaumer MH, Assessment of celiac artery compression using color-coded duplex sonography. Clinical hemorheology and microcirculation. 2020 Jul 16;
[PubMed PMID: 32675404]
[6]
Coelho JCU,Hosni AVE,Claus CM,Aguilera YSH,Abot GP,Freitas ATC,Costa MARD, Treatment of median arcuate ligament syndrome: outcome of laparoscopic approach. Arquivos brasileiros de cirurgia digestiva : ABCD = Brazilian archives of digestive surgery. 2020;
[PubMed PMID: 32428132]
[7]
Ho KKF,Walker P,Smithers BM,Foster W,Nathanson L,O'Rourke N,Shaw I,McGahan T, Outcome predictors in median arcuate ligament syndrome. Journal of vascular surgery. 2017 Jun;
[PubMed PMID: 28189355]
[8]
Selvaraj BJ,Joshi M,Weber G,Yarmush J, Celiac plexus block as a diagnostic tool in suspected pediatric median arcuate ligament syndrome. Local and regional anesthesia. 2019;
[PubMed PMID: 30881107]
[9]
Laxague F,Dreifuss N,Schlottmann F,Buxhoeveden R, Laparoscopic resolution of median arcuate ligament syndrome. Cirugia espanola. 2019 Mar 4;
[PubMed PMID: 30846189]
[10]
Patel MV,Dalag L,Weiner A,Skelly C,Lorenz J, Inability of conventional imaging findings to predict response to laparoscopic release of the median arcuate ligament in patients with celiac artery compression. Journal of vascular surgery. 2019 Feb;
[PubMed PMID: 30686339]
[11]
Berek P,Kopolovets I,Dzsinich,Sihotský V,Štefanič P,Frankovičová M, Celiac axis compression syndrome - diagnostic and surgical treatment. Rozhledy v chirurgii : mesicnik Ceskoslovenske chirurgicke spolecnosti. Summer 2018;
[PubMed PMID: 30470123]