Continuing Education Activity
This CME activity provides a comprehensive overview of fungal keratitis, a cause of corneal impairment. Participants will delve into its epidemiology, risk factors, and clinical manifestations, learning to differentiate it from bacterial and other forms of keratitis. Diagnostic approaches, including polymerase chain reaction and microscopic examination, and the latest advancements in antifungal treatments are explored. Through case studies and interactive discussions, healthcare professionals will enhance their ability to diagnose, manage, and prevent fungal keratitis, ultimately improving patient outcomes.
Objectives:
Differentiate between the clinical features of fungal keratitis and other types of corneal infections, such as bacterial keratitis.
Implement appropriate diagnostic techniques, including fluorescein staining and corneal scraping, to confirm the presence of fungal keratitis.
Apply evidence-based guidelines to select the most effective antifungal treatment options, considering factors like pathogen type and patient characteristics.
Coordinate timely referrals, consultations, and interventions, especially in complex or non-responsive cases of fungal keratitis.
Introduction
Microbial keratitis can arise from various sources, including bacteria, viruses, fungi, and protozoa. Among these, fungal keratitis (FK) is a significant contributor. Its historical documentation dates back to 1879, and its incidence has increased over the last 30 years. It accounts for 40% to 50% of all microbial keratitis cases.[1][2]
Fungal keratitis is a serious condition that demands prompt and effective intervention. Neglecting proper treatment can result in corneal destruction and endophthalmitis, leading to profound vision loss. Early diagnosis and management are essential to prevent long-term complications, including blindness.[3][4]
Over 100 fungal species have been identified as potential culprits behind fungal keratitis.[5] The predominant fungal strain responsible for these infections may exhibit variation based on geographical regions. Approximately 40% of fungal infections are secondary to trauma.
Monomorphic fungi can be classified into yeast and filamentous fungi, both of which play a role in the occurrence of fungal keratitis. The specific type of fungal variant responsible for fungal keratitis depends on several factors, including individual susceptibility, regional temperature patterns, climate conditions, geographic location, and the degree of urbanization.[6]
Fungal keratitis is notably linked to a spectrum of personal risk factors, with trauma, immunocompromised state, ocular surface disease, and contact lens usage as the most prevalent contributors. These factors not only elevate the risk of fungal keratitis but also might predispose individuals to diverse types of fungal infections, emphasizing the multifaceted nature of this condition.[6][7]
Diagnosing fungal infections as the source of keratitis can be challenging in clinical settings, often leading to delays in confirming through culture results. This makes it crucial to strongly consider the possibility of fungal keratitis, especially when specific risk factors are present. Even after diagnosis, managing the condition is challenging because many antifungal medications struggle to penetrate the cornea effectively.[8]
Etiology
Various fungal species can result in fungal keratitis, with the most common culprits being Fusarium, Aspergillus, and Candida species.[5] The causative organism of fungal keratitis may differ according to several factors, including regional temperature, climate, and urbanization.
A study in India on fungal keratitis found Aspergillus species to be the most commonly isolated species, followed by Fusarium.[9] This pattern was consistently observed in several other Indian studies, with 1 study showing that Aspergillus was responsible for more than 55% of all fungal keratitis cases, highlighting its predominant role in the Indian subcontinent.[10] However, in various other parts of the world, including southern India, distinct studies have identified Fusarium as the primary cause of fungal keratitis.[11][12]
A comprehensive study conducted in the United States over 7 years found that Fusarium was responsible for 39% of fungal keratitis, while yeasts like Candida contributed to 22% of cases.[7] Meanwhile, in central China, an extensive examination of 2065 confirmed fungal keratitis cases revealed Fuasrium as the predominant species (>50%), followed by Aspergillus (>9%) and Alternaria (>7%).[13] Similar findings were echoed in a study from northern China.[1]
Contrasting these results, research conducted in the United Kingdom highlighted Candida (57%) as the prevailing causative fungus, followed by Aspergillus (17%).[6] These geographic disparities emphasize the influence of location on the specific organism responsible for fungal keratitis.
Specific patient-related risk factors also influence the prevalence of specific fungal types causing fungal keratitis. For example, Fusarium species are more commonly associated with trauma and contact lens use,[7][14] whereas Candida species tend to be linked with ocular surface disease and topical steroid use.[6] Trauma, especially involving vegetative matter, serves as a significant risk factor for filamentary fungal infections like Fusarium, though it can also lead to bacterial infections.[13][15]
Consequently, certain occupations, particularly those in agriculture, may carry an elevated risk of microbial keratitis, including fungal keratitis.[13] The likelihood of corneal fungal infections can also increase due to inadequate personal hygiene, including poor hand hygiene and overnight contact lens wear.[16]
Corneal refractive surgeries, such as laser in situ keratomileusis (LASIK), may also rarely cause fungal keratitis, carrying the potential for grave consequences if not effectively addressed.[17] Various fungal species responsible for post-LASIK keratitis have been isolated, including Candida, Fusarium, Aspergillus, and Alternaria species.[18][19][20][21] This could be due to inappropriate operative room sterilization techniques or poor postoperative hygiene.[18]
Classification of Fungal Keratitis
Filamentous septate
Nonpigmented
Pigmented
- Cladosporium
- Curvilaria
- Alternaria
- Lasiodiplodia
Filamentous non-septate
Yeast
Epidemiology
Like most infectious diseases, the prevalence and underlying causes of fungal keratitis are influenced by geographic location and socioeconomic status. In the United States, regions with warmer climates, like Florida, exhibit a higher incidence of fungal keratitis than colder northern areas.[22][23] Fusarium, Candida, and Aspergillus are the most frequently isolated species causing FK in the USA [7], while in India, Aspergillus is the most common cause.[10] Fusarium is a prevalent cause of fungal keratitis in warm climates such as Brazil [24], while Candida may be more common in temperate climates.[23]
Filamentous fungi like Fusarium and Aspergillus spp are the prevailing fungal isolates in warmer climates, often arising from traumatic events. Epidemiological investigations in Brazil have revealed an incidence of 67% for Fusarium, 10.5% for Aspergillus, and 10% for Candida.
In the United States, a report highlights that fungal keratitis is more frequently encountered among older people, debilitated and immunocompromised populations, with Candida being the primary causative organism.
The incidence of fungal keratitis seems to be markedly higher in certain parts of the world. A comprehensive study over a decade in Hyderabad, India, examined 1360 patients with fungal keratitis within a single institution. Meanwhile, in central China, an investigation documented 2065 cases over 9 years.[13] In contrast, a study from Melbourne, Australia, recorded only 56 instances over 8 years.[25] Similarly, in a study from New York, documentation of fungal keratitis encompassed just 61 eyes over a 16-year timeframe.[26] These disparities could likely be attributed to variations in climate, environmental factors, occupational patterns, or individual behaviors.
Approximately 45 million residents in the United States are active users of contact lenses.[27] The utilization of contact lenses, when not adhering to proper practices, can elevate the risk of microbial keratitis. It is estimated that over 80% of contact lens users across diverse age groups in the USA engage in behaviors that heighten their susceptibility to contact lens-associated eye infections.[28] These risky behaviors include wearing contact lenses overnight and using tap water to clean them.
A significant outbreak of Fusarium keratitis across the United States and several other countries between 2005 and 2006. However, the outbreak's origins were eventually linked to a specific brand of multipurpose contact lens disinfecting solution. This incident underscores the importance of ongoing vigilance and scrutiny of these products and their constituents to decrease the potential for future contact lens-associated eye infections.[29]
Several studies have shown a higher incidence of fungal keratitis among young adult males, possibly due to more outdoor activities and a higher incidence of trauma. In an extensive study of Chinese patients with fungal keratitis, more than 60% were males, and more than 75% were aged between 30 and 60.[13]
Pathophysiology
The fungal pathogens causing keratitis are ubiquitous saprophytic microorganisms. Fungal keratitis often arises from fungi infiltrating the corneal stroma through defects in the corneal epithelium. This mechanism could explain the increased fungal keratitis risk associated with factors like trauma involving vegetative matter, soil or dust, contact lens usage, prior ocular surgeries, and foreign body presence, all compromising the protective epithelial layer.[30]
Trauma involving the vegetative matter can result in direct inoculation of fungal conidia on their surface into the corneal stroma leading to infection or harm of the overlying corneal epithelium, allowing fungal invasion.[1][14] The rapid proliferation of fungi subsequently triggers intensified inflammatory reactions and tissue necrosis.
Once within the tissue, fungi initiate replication within the stroma, exhibiting circumferential expansion with satellite lesions. Progressively they penetrate deeper into the stroma, breaching Descemet's membrane and gaining access to the anterior chamber. This progression may culminate in corneal perforation and endophthalmitis unless the body's natural defenses or appropriate treatment intervene effectively.[31]
Fungal infiltration can extend beyond the cornea, infiltrating the sclera and surrounding structures, potentially precipitating severe conditions such as scleritis and panophthalmitis. Fungal keratitis can also result in secondary sequelae of fungal endophthalmitis. In this scenario, the fungus retraces its course, spreading reverse from the posterior segment to Descemet's membrane, then permeating the stroma or infiltrating the corneoscleral trabeculae, traversing into the corneal channels.[32]
The avascular cornea possesses a barrier capacity and immune privilege, which renders it susceptible to colonization by fungi due to limited defense mechanisms, including dendritic cells, immune cells, and immunoglobulins. Fungi employ various tools to facilitate corneal invasion and colonization, including toxins (mycotoxins) and enzymes, including serine proteases and matrix metalloproteinases (proteolytic enzymes).[2] The resulting immune response, driven by immune cells like polymorphonuclear leukocytes within the cornea, can paradoxically contribute to additional tissue damage.[2]
Candida predominantly invades pre-existing epithelial defects, while filamentous fungi are commonly implicated in post-traumatic infections. The virulence of fungal infections depends on the fungal-produced substances and the host's response. Some fungi can flourish within the corneal stroma by delaying the release of chemotactic substances, evading the host's immune and inflammatory reactions. Candida albicans produces phospholipase A and lysophospholipase on blastospores surfaces, aiding tissue penetration. Fusarium is highly virulent and is known to penetrate the corneal stroma and Descemet's membrane.[33]
Histopathology
Gross granular infiltration within the corneal epithelium and the anterior stroma is a hallmark feature in fungal keratitis, accompanied by observable collagen destruction, coagulative necrosis, and stromal fungal infiltration upon microscopy. Notably, fungal keratitis typically exhibits milder purulent inflammatory cellular infiltration than bacterial keratitis.[34] The presence of low levels of neutrophil infiltration in the cornea is encouraging, as these cells play a pivotal role in corneal destruction while aiming to combat the causative organism.[2] In addition, lymphocytes and plasma cells are also often seen in FK.
Several staining methods can detect fungi in corneal tissue or scrapings. These include potassium-hydroxide, gram staining, Giemsa staining, lactophenol cotton blue, methenamine silver, and calcofluor white.[23] These stains facilitate the visualization of fungal hyphae and yeast cells, aiding in the early differentiation of the causative organism before culture results are available.[35] It may also allow the detection of a mixed fungal and bacterial infection.
Distinct histopathological patterns of hyphal growth have been observed, offering an additional tool for differentiating the responsible filamentous fungus. For instance, Fusarium hyphae tend to align parallel to the corneal stroma lamellae, while Aspergillus hyphae exhibit vertical growth.[36]
History and Physical
History
The history and clinical presentation of fungal keratitis may differ according to the causative organism, whether it is a filamentous fungus or yeast.[23] It is essential to exclude any history of trauma involving vegetative matter, foreign bodies, soil, or dust entering the eye.
A history of farming or fieldwork can indirectly indicate susceptibility to fungal keratitis. Other relevant history includes contact lens usage, prior ocular surgeries, corneal transplantation, alcohol consumption, malnourishment, presence of chronic debilitating conditions, HIV infection, hepatitis, diabetes mellitus, any form of steroid use, and systemic immunosuppressant treatment. A close differential is Pythium keratitis, wherein a history of mud and clay-related injury must be ruled out.[37]
Symptoms
Typically, the hallmark manifestations of microbial keratitis are evident, encompassing symptoms like blurred vision, pain, redness, discharge, gritty sensation, photophobia, excessive tearing, and blepharospasm. Notably, it's essential to recognize that signs often surpass symptoms in fungal keratitis, whereas the reverse holds for bacterial keratitis.[38]
Signs
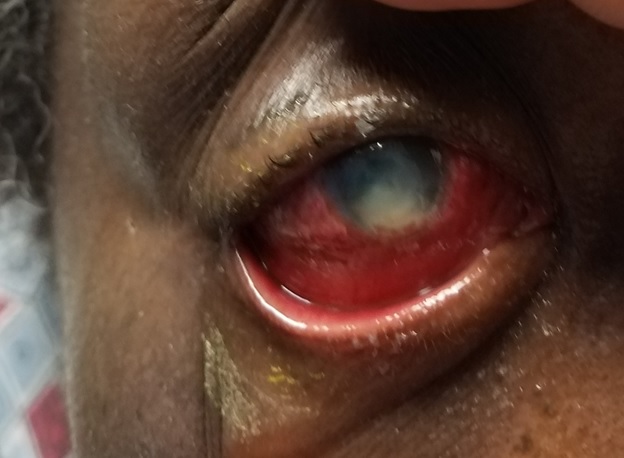
Before conducting a slit lamp examination, it is imperative to attempt a preliminary gross torch light assessment, noting prevalent clinical indicators such as lid edema, blepharospasm, matted lashes, and blepharitis (see image. Fungal Keratitis). During the slit lamp examination, a range of common clinical findings can be observed, including blepharitis, meibomian gland dysfunction, matted lashes, purulent or mucopurulent discharge, conjunctival congestion, epithelial defects, the precise location, size, depth, and extent of corneal infiltrate, distinct feathery margins, satellite lesions, stromal melt, endothelial plaque formation, thinning, descemetoceles, and perforation. Additionally, patients might display indications of inflammation within the anterior chamber, often signified by the presence of pus (hypopyon).[39]
A history of trauma involving vegetative matter is often apparent in fungal keratitis due to filamentous fungi. Clinically, patients typically present with an ulcer characterized by raised, firm slough, elongated lines of fungal hyphae extending beyond the central ulcer's border and feathery satellite stromal lesions.
Patients with fungal keratitis attributed to yeast fungi may present with a history of ocular surface disease or immunosuppression. Clinically, the presentation may resemble bacterial keratitis but with a more gradual progression. While in some cases, differentiation between fungal and bacterial causes might be possible based on clinical observations alone, this distinction is not always straightforward, and prediction of the causative fungal genus or species responsible for the condition may even be more challenging and inaccurate.[40]
Candida-induced keratitis appears as a yellow-white densely suppurative infiltrate. Keratitis due to filamentary fungus appears grey or yellow-white stromal infiltrate with indistinct fluffy margins. It is also important to note signs of scleritis and endophthalmitis in these cases, which usually develop late as a complication.[41]
Evaluation
Microscopic Examination
The laboratory assessment of fungal keratitis begins with collecting an appropriate sample for testing. These specimens undergo direct microscopic examination utilizing various stains such as 10% KOH, Gram, Giemsa, Periodic Acid- Schiff, Gomori methenamine silver stains, culture, histologic testing, and other specialized tests.[41] KOH preparation with direct microscopic examination is a highly effective and rapid diagnostic tool with over 90% sensitivity. Gram and Giemsa staining are approximately 50% sensitive. Given fungi's inclination to penetrate deep into the cornea, tissue swabbing is usually inadequate in confirming a fungal infection, necessitating the acquisition of deep corneal scrapings for a more accurate diagnosis.[42][43]
Corneal Biopsy and Polymerase Chain Reaction
Occasionally, a corneal biopsy may be needed to obtain an adequate sample. Ideally, each specimen should undergo polymerase chain reaction (PCR) and culture, especially if corneal scraping stains are negative.[42][43] Fungal cultures usually require 1 to 35 days for fungal growth to become evident. Various cultures are employed, including Sabouraud glucose neopeptone agar, blood agar, thioglycollate broth, and brain heart infusion, each serving to isolate different types of fungi.[41][43] A major disadvantage of fungal cultures is the relatively low sensitivity with false-negative results, possibly due to the small amount of material available from corneal scrapings.[42][43]
This is especially true for cases that have undergone antifungal treatment. PCR testing has been proposed as a more sensitive diagnostic method for fungal keratitis, enabling precise species identification within 4 to 8 hours. Nonetheless, it might exhibit reduced specificity, potentially leading to false-positive outcomes attributed to the amplification of non-pathogenic organisms in the sample.[23][41][43] The targets used for PCR amplification include fungal 18S rRNA and 28S rRNA sequences. While PCR can be conducted using small samples of ocular tissues or fluids like tears or aqueous humor, its application mandates specialized and costly equipment that may not always be available.
Beta-D-glucan, a component of the cell wall found in many fungal species, can be identified within the bloodstream of patients with invasive fungal infections.[44] Previous research has demonstrated that beta-d-glucan testing outpaces fungal cultures regarding speed and sensitivity when diagnosing systemic fungal infections, particularly in cases where the organisms don't thrive in blood cultures, such as Aspergillosis.[45] The presence of beta-D-glucan has the potential to be detected in tear samples from individuals affected by fungal keratitis, expediting early and prompt diagnosis.[46] Furthermore, it can also be seen in intraocular fluids of cases complicated by fungal endophthalmitis.[47]
Anterior Chamber Tap
In resistant cases, an anterior chamber tap has been advocated, mainly when an endothelial plaque is evident, as fungal infiltration is often observed to breach the endothelium.
Imaging
Non-invasive techniques, such as confocal microscopy and anterior segment optical coherence tomography (AS-OCT), can offer alternative avenues for detecting microorganisms responsible for microbial keratitis, including fungal infections, through in vivo examination techniques. Confocal microscopy can potentially reveal hyphae-like branching white lines within the infiltration area, particularly in cases associated with filamentous fungi.[48] In contrast, AS-OCT may show early localized and diffuse regions of necrotic stromal cystic spaces in cases of infection by Aspergillus species.[49] These modalities also find utility in monitoring the response to treatment as part of the follow-up process.[50]
Ophthalmic B-scan ultrasonography can be helpful in the diagnosis and follow-up of cases suspected of progressing toward endophthalmitis, especially when corneal fungal infiltration precludes examination with ophthalmoscopy.
Treatment / Management
General Measures
Hospital Admission
Hospitalization is deemed necessary for specific patient groups, including older, debilitated bedridden patients who cannot self-administer medications. Additionally, admission is warranted for cases of advanced fungal keratitis with progressive infiltration, one-eyed patients, and those originating from distant locations.[51]
Contact Lens Avoidance
It is imperative to discontinue using contact lenses in these cases promptly.[52]
Ocular Shield and Barrier
For cases at risk of or experiencing perforation, the application of a pad and bandage, along with antibiotic ointment, is essential. A transparent plastic eye shield can also be positioned between the administration of eye drops.[53]
Prophylactic Treatment and Decision to Treat
Initial, less severe corneal infiltrates may not necessitate aggressive intervention and can be managed with topical antifungals administered at a low to moderate frequency. Simultaneously, comprehensive risk management, including addressing conditions such as diabetes mellitus and refraining from contact lens use, remains crucial.
In cases where smear and culture results are pending, initiating empirical treatment is imperative to preclude the advancement of ulceration and potential complications.[54]
Scraping the Epithelium and Infiltrate
This strategy facilitates improved penetration of antifungal agents, considering their restricted ability to reach the cornea and anterior chamber. Regular removal of mucus and necrotic debris using a spatula further enhances the effectiveness of antifungal penetration.[32]
Medical Treatment
Topical Antifungals
All existing antifungal agents exhibit fungistatic properties rather than being fungicidal, necessitating a prolonged treatment period until the body's defenses can completely eradicate the fungal organism. However, instances of primary treatment failure have previously been reported in 31.3% of cases.[55]
Topical agents utilized in the treatment of FK include:
- Natamycin 5%
- First treatment of choice for NF, especially filamentous fungi [56][57]
- Amphotericin B 0.15-0.3%
- Preferred treatment of choice for yeasts
- Voriconazole 1%
- Better penetration into the eye
- Purported to be a superior alternative to natamycin
- Can be used as intrastromal or intracameral injections [58]
- Econazole 1%
- Itraconazole 1%
- Miconazole 1%
During the initial 48 hours, topical agents should be administered hourly, followed by a subsequent tapering or reduction contingent upon clinical response.
Given the predominantly fungastic nature of most antifungal agents, treatment should be continued for a minimum of 12 weeks. Candida infections warrant therapy with amphotericin B 0.15% or econazole 1%. For other cases, natamycin 5%, voriconazole 1%, clotrimazole 1% and fluconazole 2% are recommended.
Filamentous fungi infections are treated with 5% natamycin or 1% econazole, while alternative options include amphotericin B 0.15%, miconazole 1%, and voriconazole 1%.
Subconjunctival Antifungals
Subconjunctival injections of antifungal agents such as miconazole and fluconazole may be used for patients displaying suboptimal adherence to treatment or experiencing severe keratitis.[31]
Systemic Antifungals
Systemic treatment is required in severe progressive ulcers, ulcers infiltrating the limbus, scleritis, and endophthalmitis.[41] These can be found in the following forms:
- Oral
- Voriconazole 400 mg
- Itraconazole 200 mg once daily, then reduced to 100 mg once daily
- Fluconazole 200 mg twice daily
Decisions to continue therapy as based on the following biomicroscopic findings:
- A decrease in pain and size of infiltrate
- A reduction of satellite lesions
- A reduction in the density of suppuration
- Blunting of the infiltrated edges
- A decrease in inflammation in the anterior chamber
- Continued reepithelialization
Recently published clinical trials have reported equal or inferior efficacy of topical 1% voriconazole (reconstituted from injection vial) compared with topical 5% natamycin eye drops for treating fungal keratitis, especially those caused by Fusarium. These results, however, are contradictory to experimental and in vitro data. Sharma et al conducted a study involving 118 patients, 58 receiving voriconazole and 60 treated with natamycin. Notably, while the proportion of ulcers that were healing or resolving appeared similar on day 7 (natamycin 35/54, 65%; voriconazole 34/50, 68%), the final analysis unveiled a significant divergence, Specifically at the last visit, the percentage of patients exhibiting healed corneal ulcers were notably higher in the natamycin-treated group (50/56, 89.2%) compared to the voriconazole-treated group (34/51, 66.6%; p=0.005).[59]
In 2015, a systematic review of medical treatments for fungal keratitis was conducted by the Cochrane Database, concluding that natamycin may be more effective than voriconazole in treating fungal keratitis; however, most studies were underpowered with variable quality.[60] The encompassed randomized controlled trials featured a diverse array of comparisons, including topical 5% natamycin versus topical 1% voriconazole, topical 1% voriconazole vs. intrastromal voriconazole, and topical 1% natamycin versus topical 2% econazole.
Role of Antibiotics
To preempt potential bacterial co-infection, broad-spectrum antibiotics are recommended. Tetracyclines such as doxycycline 100 mg twice daily may be required due to their anti-collagenase properties.[61]
Corticosteroids
Steroids are unequivocally contraindicated during active fungal keratitis due to the potential to foster rapid fungal proliferation and exacerbate vision-threatening keratitis. The functional role of steroids in fungal keratitis is post-therapeutic keratoplasty (TPK) once the eye is free of infection.
Steroids are started cautiously around 3 to 4 weeks after TPK and under close supervision. Predominantly, topical agents such as prednisolone 1% or dexamethasone 0.1% are favored due to their favorable anterior chamber penetration.
Initial dosing entails a low frequency of 3 times a day during the initial week, transitioning to a slow taper over 3 months, fostering graft survival and preventing rejection episodes. There is no role of systemic steroids in fungal keratitis.[62]
Surgical Treatment
Superficial Keratectomy
A superficial keratectomy is necessary to debulk the ulcer effectively.[63]
Therapeutic Keratoplasty
Patients who do not respond favorably to medical therapy may require surgical intervention, including therapeutic penetrating and lamellar keratoplasty.[13][64] Therapeutic keratoplasty can be associated with complications, including recurrence of infection, endophthalmitis, and graft rejection.[64]
Anterior Chamber Wash
In cases where the standard treatment approach proves ineffective and is met with non-responsiveness, an anterior chamber wash becomes necessary, particularly when accompanied by the presence of hypopyon and endothelial exudates.
Intracameral and Intrastromal Injection of Voriconazole
When ulcers display a slow response to topical treatment, manifest as full-thickness ulcers, exhibit a progressive course, or demonstrate resistance to conventional therapies, intracameral and intrastromal voriconazole becomes imperative for enhanced management.[65]
Corneal Collagen Crosslinking
A recently available treatment modality is corneal collagen crosslinking which may sometimes be useful in medically resistant corneal ulcers or even in some early cases of fungal infection.[66][67][68] However, a recent randomized controlled clinical trial did not demonstrate any benefit from this approach in fungal keratitis and indicated an elevated incidence of complications.[69] Conjunctival flaps can also rarely be used but may increase the incidence of graft rejection following keratoplasty due to corneal vascularization.[13]
To save the eye, a tectonic or therapeutic keratoplasty is usually required in corneal perforation. Cases complicated by endophthalmitis may require intravitreal antifungal injections or even necessitate pars plana vitrectomy, which can be performed using endoscopy if extensive corneal infiltration impedes posterior segment visualization.[70][71][72] However, enucleation may eventually be the last resort in cases where the eye is rendered blind and painful, with uncontrollable inflammation.
Healing of fungal keratitis may result in central corneal scarring and opacification, which may require penetrating or lamellar keratoplasty to restore visual acuity.
Repeat Therapeutic Keratoplasty
Repeated therapeutic keratoplasty in non-resolving graft infections should be attempted to clear the infective focus.[73]
Temporary Tarsorrhaphy
After repeat keratoplasty, if there is persistent graft infection, repeat keratoplasty (third graft) along with temporary tarsorrhaphy should be performed to allow resolution of infection.[74]
Evisceration
In cases with non-resolving endophthalmitis or panophthalmitis, the option of evisceration is considered, contingent upon obtaining appropriate consent from the patient and their next of kin.[75]
Differential Diagnosis
The differential diagnosis for fungal keratitis includes the following:
- Bacterial keratitis [76]
- Pythium keratitis [77][78][79][80][81][82]
- Viral keratitis
- Neurotrophic keratitis
- Acanthamoeba keratitis
- Necrotizing herpetic keratitis
- Exposure keratopathy
Prognosis
The prognosis for fungal keratitis hinges on several factors, including the causative organism, the depth and extent of the infection, the development of complications, and the promptness of treatment initiation. While certain patients may achieve microbiological cure through topical antifungal agents, others might necessitate therapeutic keratoplasty.[83]
The integrity of the Descemet membrane, positioned in the interior basement membrane near the aqueous humor, is usually impermeable to bacteria; however, fungal hyphae can breach this barrier, culminating in endophthalmitis-- a rare yet grave consequence of fungal keratitis.
Approximately 30% of fungal infections do not respond to drug therapy, potentially culminating in corneal perforation. Successful resolution of fungal keratitis usually results in central corneal scarring and opacification, which may require penetrating or lamellar keratoplasty to restore visual acuity.[84]
Complications
The complications arising from fungal keratitis encompass the following:
- Non-resolving epithelial defect
- Corneal perforation
- Corneal melting
- Corneal scarring
- Secondary glaucoma
- Iris neovascularization
- Peripheral anterior synechiae
- Posterior synechiae
- Scleritis
- Endophthalmitis
- Panophthalmitis
- Complicated cataract
- Vitritis
- Vitreous membranes
- Permanent blindness
- Phthisis bulbi
- Atrophic bulbi
Postoperative and Rehabilitation Care
Following therapeutic or tectonic keratoplasty, each patient necessitates meticulous management, consistent and timely care, and vigilant follow-up. Initially, the commencement of topical antifungal agents post-TPK is advised, typically in low doses, around 6 times daily alongside adjuvant medications. Subsequent follow-up appointments are recommended on postoperative days 1, 5, 14, 21, and 28, with later intervals of every 2 weeks or months based on the patient's clinical condition.
Topical steroids should be introduced after 3 to 4 weeks, depending on the clinical picture.[85] If the preoperative culture and button culture reports are negative and there are no signs of infection, topical steroids can be started after 3 weeks post-TPK. Conversely, if either or both reports indicate positivity, the initiation of topical steroids can be deferred to 4 weeks post-TPK.[86]
It is crucial to convey to patients the significance of diligent follow-up and consistent adherence to prescribed medication schedules. Patients should be provided with a clear understanding of the potential complications associated with keratoplasty and the prognosis specific to their case. Open communication should prepare patients for the possibility of undergoing repeat keratoplasty if warranted. It's worth noting that the prognosis tends to diminish with each subsequent keratoplasty.[87]
Consultations
Patients presenting with a corneal ulcer should be referred to a cornea and external disease specialist with the expertise to diagnose and accurately tailor appropriate management. The distinctive features of the ulcer can be readily identified by the cornea specialist through clinical evaluation.
Should the condition give rise to secondary glaucoma, referral to a glaucoma specialist is recommended for initiating antiglaucoma medications or considering procedures like trabeculectomy or glaucoma drainage devices. If the patient develops complex cataract issues, timely intervention by a cataract and IOL or cornea specialist proficient in cataract surgery becomes imperative. Expert treatment from a retina specialist is essential for instances where retinal complications emerge, such as vitritis, endophthalmitis, panophthalmitis, and retinal or choroidal detachment.
Deterrence and Patient Education
Patients should be educated about using contact lenses and the appropriate cleaning products to minimize the risk of contact lens-associated corneal infections. Proper hygienic measures should be emphasized, including frequent hand washing before handling contact lenses and avoiding overnight contact lens wear.[88]
Enhancing Healthcare Team Outcomes
Most patients with fungal keratitis are initially encountered in the emergency department or through primary care providers. To ensure a prompt referral to an ophthalmologist and avoid delays that might result in vision impairment or permanent blindness, an interprofessional approach to diagnosis and treatment is crucial. While most patients with fungal keratitis can be managed as outpatients, it's important to note that they require a sustained course of antifungal therapy spanning 12 to 16 weeks.[30]
Patient education is vital in preventing fungal keratitis. Contact lens wearers must be educated about proper hand hygiene, the correct cleaning solutions, and the importance of avoiding sleeping or swimming while wearing lenses. Regular follow-up appointments with opticians or ophthalmologists are essential for contact lens wearers. They should be informed about the symptoms of eye infections, such as pain, redness, or vision loss, and be advised to seek immediate ophthalmologic consultation if these symptoms arise.
Ophthalmology nurses are pivotal in patient education, facilitating follow-up appointments, and maintaining effective communication among the healthcare team. Pharmacists also contribute by ensuring appropriate medication dosages, identifying potential drug interactions, and reviewing proper administration methods with patients.[89]
Close follow-up throughout the treatment period is essential to monitor the condition's progression and ensure its stabilization. The eventual outcome is influenced by various factors, including the patient's overall health, immune system status, and other comorbidities. Patients with mild infections that are promptly treated tend to have favorable outcomes. However, for those with infections extending into the sclera, the prognosis becomes guarded. Available data indicates that at least 30% of patients with fungal keratitis develop corneal perforation or show inadequate response to drug therapy.[90][91]