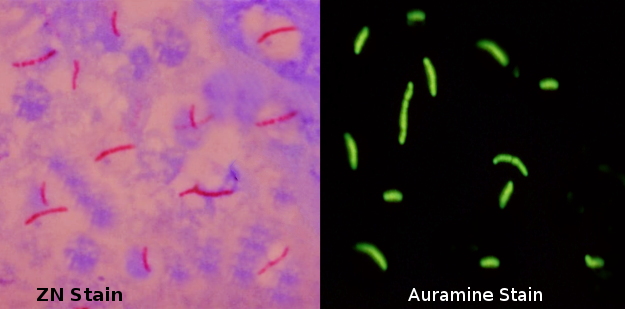
[1]
Reynolds J, Moyes RB, Breakwell DP. Differential staining of bacteria: acid fast stain. Current protocols in microbiology. 2009 Nov:Appendix 3():Appendix 3H. doi: 10.1002/9780471729259.mca03hs15. Epub
[PubMed PMID: 19885935]
[3]
Ryu YJ. Diagnosis of pulmonary tuberculosis: recent advances and diagnostic algorithms. Tuberculosis and respiratory diseases. 2015 Apr:78(2):64-71. doi: 10.4046/trd.2015.78.2.64. Epub 2015 Apr 2
[PubMed PMID: 25861338]
Level 3 (low-level) evidence
[4]
Mosissa L, Kebede A, Mindaye T, Getahun M, Tulu S, Desta K. External quality assessment of AFB smear microscopy performances and its associated factors in selected private health facilities in Addis Ababa, Ethiopia. The Pan African medical journal. 2016:24():125. doi: 10.11604/pamj.2016.24.125.7459. Epub 2016 Jun 9
[PubMed PMID: 27642463]
Level 2 (mid-level) evidence
[5]
Ugarte-Gil C, Elkington PT, Gotuzzo E, Friedland JS, Moore DAJ. Induced sputum is safe and well-tolerated for TB diagnosis in a resource-poor primary healthcare setting. The American journal of tropical medicine and hygiene. 2015 Mar:92(3):633-635. doi: 10.4269/ajtmh.14-0583. Epub 2014 Dec 22
[PubMed PMID: 25535311]
[6]
Zar HJ, Tannenbaum E, Apolles P, Roux P, Hanslo D, Hussey G. Sputum induction for the diagnosis of pulmonary tuberculosis in infants and young children in an urban setting in South Africa. Archives of disease in childhood. 2000 Apr:82(4):305-8
[PubMed PMID: 10735837]
[7]
Yoon SH, Lee NK, Yim JJ. Impact of sputum gross appearance and volume on smear positivity of pulmonary tuberculosis: a prospective cohort study. BMC infectious diseases. 2012 Aug 1:12():172
[PubMed PMID: 22853561]
[8]
Warren JR, Bhattacharya M, De Almeida KN, Trakas K, Peterson LR. A minimum 5.0 ml of sputum improves the sensitivity of acid-fast smear for Mycobacterium tuberculosis. American journal of respiratory and critical care medicine. 2000 May:161(5):1559-62
[PubMed PMID: 10806154]
[10]
Van Rie A, Fitzgerald D, Kabuya G, Van Deun A, Tabala M, Jarret N, Behets F, Bahati E. Sputum smear microscopy: evaluation of impact of training, microscope distribution, and use of external quality assessment guidelines for resource-poor settings. Journal of clinical microbiology. 2008 Mar:46(3):897-901. doi: 10.1128/JCM.01553-07. Epub 2008 Jan 3
[PubMed PMID: 18174302]
Level 2 (mid-level) evidence
[11]
Reji P, Aga G, Abebe G. The role of AFB microscopy training in improving the performance of laboratory professionals: analysis of pre and post training evaluation scores. BMC health services research. 2013 Oct 7:13():392. doi: 10.1186/1472-6963-13-392. Epub 2013 Oct 7
[PubMed PMID: 24099153]
[12]
Van Deun A, Hossain MA, Gumusboga M, Rieder HL. Ziehl-Neelsen staining: theory and practice. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2008 Jan:12(1):108-10
[PubMed PMID: 18173887]
[13]
Hooja S, Pal N, Malhotra B, Goyal S, Kumar V, Vyas L. Comparison of Ziehl Neelsen & Auramine O staining methods on direct and concentrated smears in clinical specimens. The Indian journal of tuberculosis. 2011 Apr:58(2):72-6
[PubMed PMID: 21644393]
[14]
Ba F, Rieder HL. A comparison of fluorescence microscopy with the Ziehl-Neelsen technique in the examination of sputum for acid-fast bacilli. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 1999 Dec:3(12):1101-5
[PubMed PMID: 10599014]
[15]
Singh NP, Parija SC. The value of fluorescence microscopy of auramine stained sputum smears for the diagnosis of pulmonary tuberculosis. The Southeast Asian journal of tropical medicine and public health. 1998 Dec:29(4):860-3
[PubMed PMID: 10772577]
[16]
Noori MY, Ali Z, Ali Wahidi SA, Mughal MN, Sharafat S, Masroor M, Khanani MR. False negativity in AFB Smear microscopy: An insight into the caveats of the most widely used screening tool for tuberculosis. JPMA. The Journal of the Pakistan Medical Association. 2016 Sep:66(9):1116-1119
[PubMed PMID: 27654731]
[17]
Nicol MP, Whitelaw A, Wendy S. Using Xpert MTB/RIF. Current respiratory medicine reviews. 2013 Jun:9():187-192
[PubMed PMID: 24089608]
[18]
. Xpert MTB/RIF Implementation Manual: Technical and Operational ‘How-To’; Practical Considerations. 2014:():
[PubMed PMID: 25473699]
[19]
Desikan P. Sputum smear microscopy in tuberculosis: is it still relevant? The Indian journal of medical research. 2013 Mar:137(3):442-4
[PubMed PMID: 23640550]
[20]
Lawn SD, Nicol MP. Xpert® MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future microbiology. 2011 Sep:6(9):1067-82. doi: 10.2217/fmb.11.84. Epub
[PubMed PMID: 21958145]
[21]
Angra P, Ridderhof J, Tahseen S, Van Deun A. Read the new microscopy handbook: even the Ziehl-Neelsen technique has changed. The international journal of tuberculosis and lung disease : the official journal of the International Union against Tuberculosis and Lung Disease. 2016 Apr:20(4):567. doi: 10.5588/ijtld.16.0009. Epub
[PubMed PMID: 26970169]
[22]
Abdelaziz MM, Bakr WM, Hussien SM, Amine AE. Diagnosis of pulmonary tuberculosis using Ziehl-Neelsen stain or cold staining techniques? The Journal of the Egyptian Public Health Association. 2016 Mar:91(1):39-43. doi: 10.1097/01.EPX.0000481358.12903.af. Epub
[PubMed PMID: 27110859]
[23]
Minion J, Shenai S, Vadwai V, Tipnis T, Greenaway C, Menzies D, Ramsay A, Rodrigues C, Pai M. Fading of auramine-stained mycobacterial smears and implications for external quality assurance. Journal of clinical microbiology. 2011 May:49(5):2024-6. doi: 10.1128/JCM.00507-11. Epub 2011 Mar 23
[PubMed PMID: 21430105]
Level 2 (mid-level) evidence
[24]
Mekonen A, Ayele Y, Berhan Y, Woldeyohannes D, Erku W, Sisay S. Factors which contributed for low quality sputum smears for the detection of acid fast bacilli (AFB) at selected health centers in Ethiopia: A quality control perspective. PloS one. 2018:13(6):e0198947. doi: 10.1371/journal.pone.0198947. Epub 2018 Jun 20
[PubMed PMID: 29924828]
Level 2 (mid-level) evidence
[25]
Aouam K, Chaabane A, Loussaïef C, Ben Romdhane F, Boughattas NA, Chakroun M. [Adverse effects of antitubercular drugs: epidemiology, mechanisms, and patient management]. Medecine et maladies infectieuses. 2007 May:37(5):253-61
[PubMed PMID: 17336011]
[26]
Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert opinion on drug safety. 2006 Mar:5(2):231-49
[PubMed PMID: 16503745]
[27]
Daftary A, Frick M, Venkatesan N, Pai M. Fighting TB stigma: we need to apply lessons learnt from HIV activism. BMJ global health. 2017:2(4):e000515. doi: 10.1136/bmjgh-2017-000515. Epub 2017 Oct 31
[PubMed PMID: 29225954]
[28]
Morisky DE, Malotte CK, Ebin V, Davidson P, Cabrera D, Trout PT, Coly A. Behavioral interventions for the control of tuberculosis among adolescents. Public health reports (Washington, D.C. : 1974). 2001 Nov-Dec:116(6):568-74
[PubMed PMID: 12196616]