Introduction
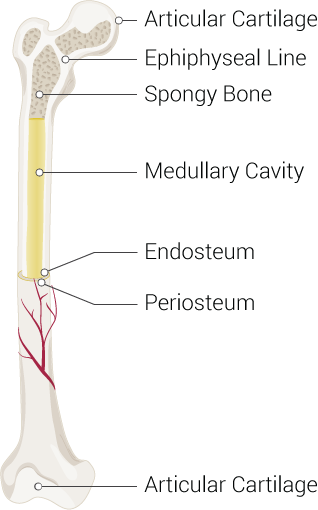
Bones are often considered static structures that only offer structural support (see Image. Parts of a Long Bone). However, bones have many functions, like other organ systems. Besides serving as a framework for soft tissue, bones permit locomotion, protect vital organs, facilitate breathing, play a role in electrolyte homeostasis, and are the sites of hematopoiesis. Bone remodeling continues throughout life, driven by physiologic demands.
The skeletal system can respond to increased mechanical stress by activating osteogenesis—the bone formation process. This ability is evident in how resistance training shapes the body. Resistance exercise has proven to be a viable therapeutic option for osteosarcopenia or age-related bone and muscle loss.[1]
Bones adapt in response to both external and internal stimuli. Unlike other organs, these hard structures may break when subjected to excessive force but regenerate without fibrosis or scarring.
Human infants typically have 270 bones, fusing into 206 to 213 bones in the human adult. The variability in number arises from variations in the anatomy of some bony locations. Bones differ in size, shape, and strength, depending on function. This topic focuses on bone anatomy and function and the clinical conditions involving these structures.
Structure and Function
Bone Structure
From a histological perspective, bones are highly specialized connective tissues that can remodel based on exogenous demand. The cell primarily responsible for building bones is the osteoblast, which secretes a collagen-rich fluid known as osteoid. Ground substance, composed primarily of osteocalcin and chondroitin sulfate, is also present in osteoid.
Bone hardening requires mineralization, a process that makes bones the body's main calcium and phosphate reservoir. These ions are typically obtained from the diet, eg, dairy products.[2] However, calcium and phosphate can easily deplete if not stored in bones. Mineralization transforms the osteoblast into the mature bone cell, the osteocyte.
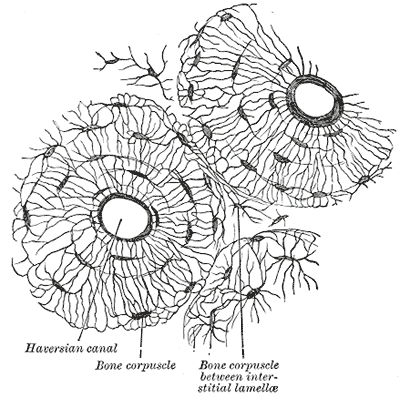
The osteon is the bone's functional unit (see Image. Osteon). Osteocytes in the osteon are typically found in lacunae arranged concentrically around a central opening, the Haversian canal. Canaliculi are osteocyte processes interconnected by gap junctions, allowing the mature bone cells to communicate and exchange cytoplasmic contents.
The osteon's blood supply originates from the highly vascularized periosteum, the soft tissue covering the bone's outer surface. Periosteal blood vessels reach the Haversian canal via the endosteum-lined Volkmann canals, the perpendicular channels traversing the osteon. Arteries emanating from the Haversian canal supply the osteocytes in the inner two-thirds of cortical bone and the marrow cavity.
From a gross anatomical perspective, most bones have a well-organized, compact outer shell comprised of osteons known as the cortex. Bones' inner portions are meshlike and are called "trabecular bones," "spongy bones," or "cancellous bones." The meshlike quality of the inner surface reduces the impact of strong external forces and leaves space for hematopoiesis. Osteons run parallel to the bone shaft.
The ratio of cortical to trabecular bone in healthy adults is approximately 80:20. The vertebrae are the only bones without a true cortex and are entirely covered by a dense trabecular network. The periosteum covers all bones, providing innervation, circulation, and nutrition, especially to the outer third bone segment.
Bone Classification
Bones can be classified into long, short, and flat bones.
Long bones evolve via endochondral ossification. Examples of long bones are the femur and phalanges. These bones vary in size and are typically tubular. Long bones have 3 distinct anatomical zones:
- Diaphysis, aka shaft: Contains the bone medulla, which houses yellow bone marrow.
- Epiphysis: Tip of the long bone, typically responsible for articulation. The epiphysis is also the primary source of red bone marrow in long bones, the site of erythropoiesis.
- Metaphysis: The region between the diaphysis and epiphysis that contains the epiphyseal plate in children. Epiphyseal plates are responsible for linear bone growth and remain cartilaginous until after puberty. After ossification, the metaphysis becomes primarily responsible for transferring mechanical loads from the epiphysis to the diaphysis.
Short bones also evolve by endochondral ossification but are smaller and take on different shapes. Examples of short bones are the carpal bones.
Flat bones form by intramembranous ossification and have unique, plate-like shapes. Examples include the sternum and cranial bones.
Bone Cells
Pluripotent mesenchymal stem cells form the osteoblasts, which later become the osteocytes. During bone remodeling, osteocyte apoptosis sends signals to marrow osteoblasts to activate osteoclast formation. Osteoclasts are myeloid-derived cells that initiate bone resorption.[16]
Bone Composition
The bone extracellular matrix comprises collagen type I and crystals of the calcium phosphate mineral, calcium hydroxyapatite. The osteon contains the osteocytes, which are crucial to bone integrity and the remodeling process. Cement lines, aka cement sheaths, delineate the osteons and have a higher mineral density than other parts of the bone matrix.[17]
Bone Remodeling
Bone undergoes constant remodeling in response to stress and hormonal control. Bone is resorbed under conditions of reduced stress, leading to bone loss. Thus, bedridden patients and astronauts on prolonged space travel develop weaker bones over time.
During remodeling, osteocyte apoptosis signals osteoblasts to induce osteoclast differentiation from myeloid cells. Osteoclasts remove old osteocytes, initiate bone resorption, and form new osteoblasts. New osteocytes arise from new osteoblasts.
Osteoclasts are multinucleated cells found in Howship lacunae on the bone's inner surface. The osteoclasts' outer membranes contain the receptor activator of nuclear factor-kappa B (RANK). This receptor is activated by the osteoblasts' product, the receptor activator of nuclear factor-kappa B ligand (RANKL). Another osteoblastic secretion, osteoprotegerin, disrupts RANK-RANKL interaction to prevent osteoclast differentiation.
Osteoclasts use carbonic anhydrase and collagenase to break down the bone matrix. Carbonic anhydrase is a proton-producing enzyme that acidifies the bone matrix. Collagenase is an enzyme that degrades collagen.[18]
Estrogen increases osteoblastic activity. Reduced estrogen levels in postmenopausal women put them at risk of bone loss, osteoporosis, and fractures.
Embryology
Most bones arise from the mesoderm. Middle ear ossicles and some craniofacial bones come from neural crest cells. Long and short bones form from cartilaginous precursors and later undergo endochondral ossification. Flat bones evolve differently and develop by intramembranous ossification.
During intramembranous ossification, osteoblasts produce bone spicules, which form trabeculae. Woven bone arises from merging trabeculae. Periosteum comes from the mesenchymal cells surrounding the trabeculae. Osteogenic cells from the periosteum grow along the woven bone's surface and become lamellar bone after mineralization.
Blood Supply and Lymphatics
Long bones have a nutrient artery that enters the midshaft region of the bone. In the marrow cavity, the nutrient artery divides into longitudinal branches that travel toward each bone tip. Nearly all long bones have a nutrient artery that passes through the nutrient foramen in the middle third of the diaphysis. Some may have two nutrient arteries.
The periosteum envelops the bone surface. Volkmann canals connect the blood vessels of the periosteum to those inside the Haversian canals and adjacent osteons. Small periosteal branches supply most compact bones. Stripping off the periosteum may lead to bone cell death.
The epiphyses receive their own blood supply from the epiphyseal arteries, which arise from articular anastomoses.
Nerves
Periosteal nerves are mostly pain fibers. The pain afferents carry signals to spinal nuclei via the dorsal root ganglia. Efferent innervation on the periosteum and inside the bone comes from the sympathetic nervous system, which regulates blood flow in these areas. The sympathetic cell bodies are located in the spinal cord's intermediolateral column, and their axons form the sympathetic ganglia. Postganglionic fibers pass via the gray rami communicantes to the branches of the cervical, brachial, or lumbosacral plexuses. Fibers from these plexuses reach the bone.
Surgical Considerations
Fractures must be surgically repaired to ensure proper healing. Otherwise, malunion or nonunion can occur, causing mobility problems. Most pediatric fractures heal spontaneously or by splinting due to young bone's malleability. However, pediatric epiphyseal fractures may result in growth stunting and long bone deformities.[19] Adult fractures may be repaired by nails, screws, and plates.
Clinical Significance
Bone Fractures
Bone fractures vary in severity. Simple fractures may heal with supportive measures or closed procedures, such as splinting, casting, and closed reduction. Severe injuries typically require open reduction aided by nails, plates, and screws.[3]
Augmented reality is currently being tested as a surgical simulation training tool for orthopedic surgeons.[4] Additionally, novel treatments, such as cementless total knee replacements, are being developed to improve post-surgical outcomes.[5]
Vertebral wedge fracture and "dowager's hump"
Vertebrae have features of both cortical and trabecular bones. The outer surface of the vertebral body is composed of compact bone. Meanwhile, the inner portion contains thousands of trabeculae oriented in different directions. A "wedge fracture" occurs when the compact vertebral pedicles and processes remain intact while the inner trabecular segment gets damaged by a compressive force. Wedge fractures in the superior spine can lead to kyphosis, or the excessive forward-bending of the upper thorax, and a "dowager's hump" posterior to the base of the cervical spine.
A similar mechanism underlies vertebral wedge fractures of the lumbar vertebrae, resulting in distortion of the lumbar and thoracic curvatures.
Osteosarcoma
Osteosarcoma is a highly aggressive bone malignancy, the 3rd most common adolescent cancer after leukemia and lymphoma.[6] This condition most commonly affects patients in the 2nd and 3rd decades of life, though it can occur in younger children and older adults. Osteosarcoma involves malignant bone-forming primitive mesenchymal cells. Primary osteosarcoma most commonly affects the long bones, particularly those of the leg. Symptoms include nighttime pain, reduced range of motion, and swelling of the nearby joints.[7]
Lung metastases and post-therapeutic recurrence are frequent in osteosarcoma cases. M2-like tumor-associated macrophages (TAMS) in the tumor stroma increase the risk of pulmonary metastasis and have a poor prognosis.[8][9][10] This condition requires multidisciplinary care. Treatment usually involves chemotherapy, surgery, and sometimes, radiation. Osteosarcoma's 5-year survival rate for osteosarcoma is 60% to 70%. [11]
Dendritic cell immunotherapy is a novel intervention that uses the dendritic cells' immunomodulatory effects to eliminate cancer tissue.[12][13][14] Cryoimmunology, which combines tumor cryoablation and immunotherapy, is another modality that shows promise in osteosarcoma treatment.[15]
Other Issues
Other clinical issues that may arise within the skeletal system are the following:
- Infection: Osteomyelitis is a bacterial bone infection that can develop from inoculation or direct or hematogenous spread. Staphylococcus aureus is the most common causative agent.[20]
- Avascular necrosis: The condition occurs when the bone's blood supply is compromised. Necrosis may lead to nonunion. The femoral head and scaphoid are particularly susceptible to avascular necrosis.
- Osteoporosis: This term refers to bone weakness due to loss of density. The condition is common in older patients, especially postmenopausal women.
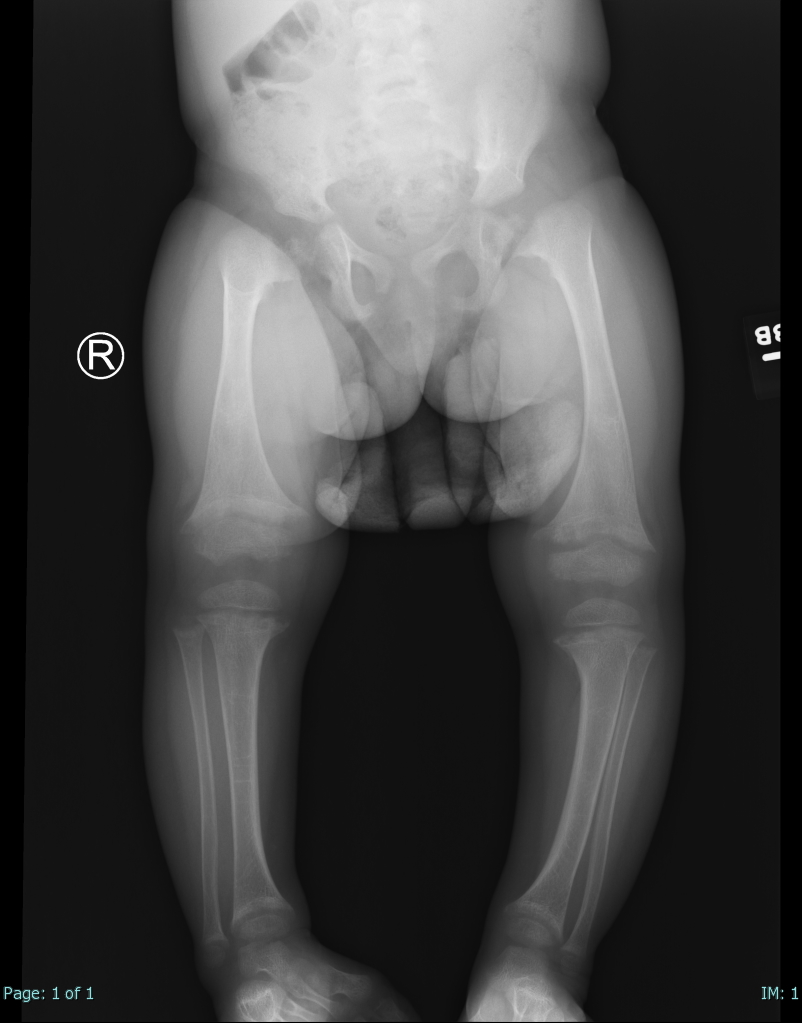
- Vitamin D deficiency: Insufficiency of vitamin D levels can lead to rickets in children and osteomalacia in adults. Vitamin D is critical to calcium and phosphate metabolism. Osteoid will not mineralize properly without this prohormone. Bone weakening and pain are characteristic (see Image. Rickets).
- Craniofacial malformations: These conditions arise from disordered neural crest cell migration.