Continuing Education Activity
Hidradenitis suppurativa (HS), known as acne inversus, presents as a chronic inflammatory skin condition characterized by painful lesions such as deep-seated nodules, abscesses, draining tracts, and fibrotic scars. These manifestations primarily emerge in intertriginous areas and regions abundant in apocrine glands, notably affecting sites like the axillary, groin, perianal, perineal, and inframammary regions. The impact of HS extends beyond physical discomfort, with associated pain, drainage, malodor, and scarring often leading to profound negative psychosocial implications for affected individuals.
This CME activity explores the etiology, clinical presentation, complications, and therapeutic strategies related to HS, highlighting the crucial role of an interprofessional team in its management. Treatment approaches span a spectrum contingent upon severity, encompassing topical and systemic antibiotics, hormone therapy, immune modulators, and surgical interventions to alleviate symptoms, mitigate disease progression, and improve the overall quality of life for those grappling with this challenging dermatological condition.
Objectives:
Determine the triggers for hidradenitis suppurativa (HS).
Identify the clinical presentation of HS.
Implement a treatment plan for HS.
Strategize modalities for improved care coordination among interprofessional team members to enhance outcomes for patients affected by HS.
Introduction
Hidradenitis suppurativa (HS), also called acne inversus, is a chronic inflammatory skin condition with lesions including deep-seated nodules and abscesses, draining tracts, and fibrotic scars. These lesions most commonly occur in intertriginous areas and areas rich in apocrine glands. Among the most common are the axillary, groin, perianal, perineal, and inframammary locations. Treatment varies based on severity and can include topical and systemic antibiotics, hormone therapy, immune modulators, and surgery. Because of the associated pain, sensitive locations, drainage, odor, and scarring, this condition may have a negative psychosocial impact.[1]
Etiology
The etiology of HS appears to have genetic, environmental, and behavioral influences.[2] Thirty-three percent to 40% of individuals with HS report an affected first-degree relative, suggesting a hereditary component with an autosomal dominant transmission pattern. Researchers have identified a mutation of the gamma-secretase Notch signaling pathway in a small subset of affected families.[3]
Environmental and behavioral factors also contribute to the development of HS. Individuals with HS are more commonly overweight or obese. Obesity leads to greater intertriginous surface area and skin friction, increased sweat production and retention, and hormonal changes leading to relative androgen excess, all associated with HS. Metabolic syndrome is more common in obese individuals and thus is also seen more commonly in HS.[4]
Smoking is also prevalent among those diagnosed with HS. Causation is unclear; however, nicotine may cause increased follicular plugging. As with obesity, disease progression and severity are worse in those who smoke.[5]
The influence of hormones can be seen in HS. There is a greater prevalence in females than males, with the age of primary occurrence most commonly between puberty and menopause. In addition, there are fluctuations in acute symptomatic episodes and severity with menstrual cycles and exogenous hormones.[6]
Epidemiology
The estimated prevalence of HS ranges from under 1% to 4%. These numbers are likely underestimated because of underreporting and misdiagnosis. The onset of the condition is commonly between puberty and age 40, most frequently from age 21 to 29. Women are more commonly affected than men in an approximate ratio of 3 to 1. There is a lack of evidence to show a pattern of racial or ethnic predilection.[7]
Pathophysiology
The pathologic process of HS begins when a defective hair follicle becomes occluded and ruptures, spilling its contents, including keratin and bacteria, into the surrounding dermis. A chemotactic inflammatory response by surrounding neutrophils and lymphocytes can lead to abscess formation and subsequent destruction of the pilosebaceous unit and other adjacent structures. Other possible contributors to pathology include abnormal antimicrobial peptides, abnormal secretion of apocrine glands, abnormal invaginations of the epidermis leading to tract formation, and deficient numbers of sebaceous glands.[8][9]
Immunological abnormalities have also been observed. Elevated levels of inflammatory cytokines, including tumor necrosis factor-alpha and various interleukins, have been detected in the lesions of HS and provide possible targets for emerging treatments. Bacteria do not appear to be causative. Aspirate from an unruptured lesion typically yields a sterile culture. However, bacterial infection and colonization during the process can secondarily worsen HS.[2]
History and Physical
Because the early stages of HS are often mistaken for other conditions, the average delay in the correct diagnosis is 7 years.[2] Clinical diagnosis requires recognition of the morphology (deep, inflamed, painful nodules, sinus tracts, scars), the location (intertriginous areas, apocrine gland-containing areas), and the chronicity of the disease process (prolonged course with periods of activity and remission).
Up to half of individuals with HS will report a prodromal syndrome involving burning, stinging, pain, pruritus, warmth, or hyperhidrosis in the area 12 to 48 hours before the appearance of a lesion. Triggers can include menstruation, weight gain, stress, hormonal changes, excessive heat, and perspiration. On presentation, individuals are typically well-appearing and afebrile unless secondary infection or advanced disease is present.[10]
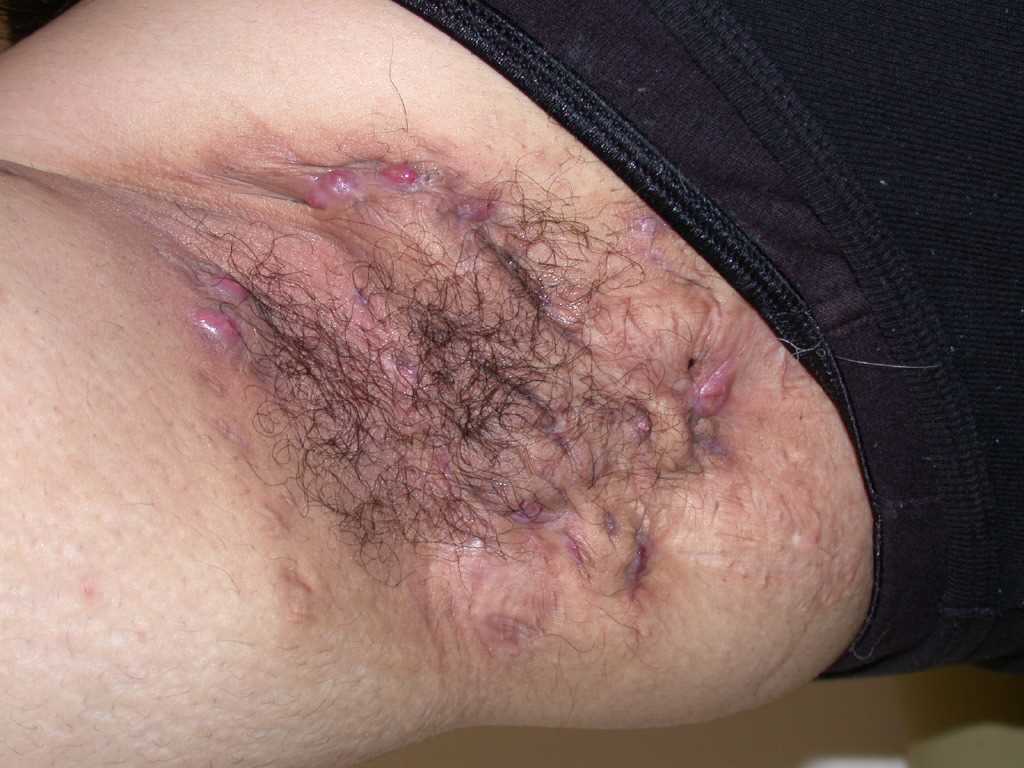
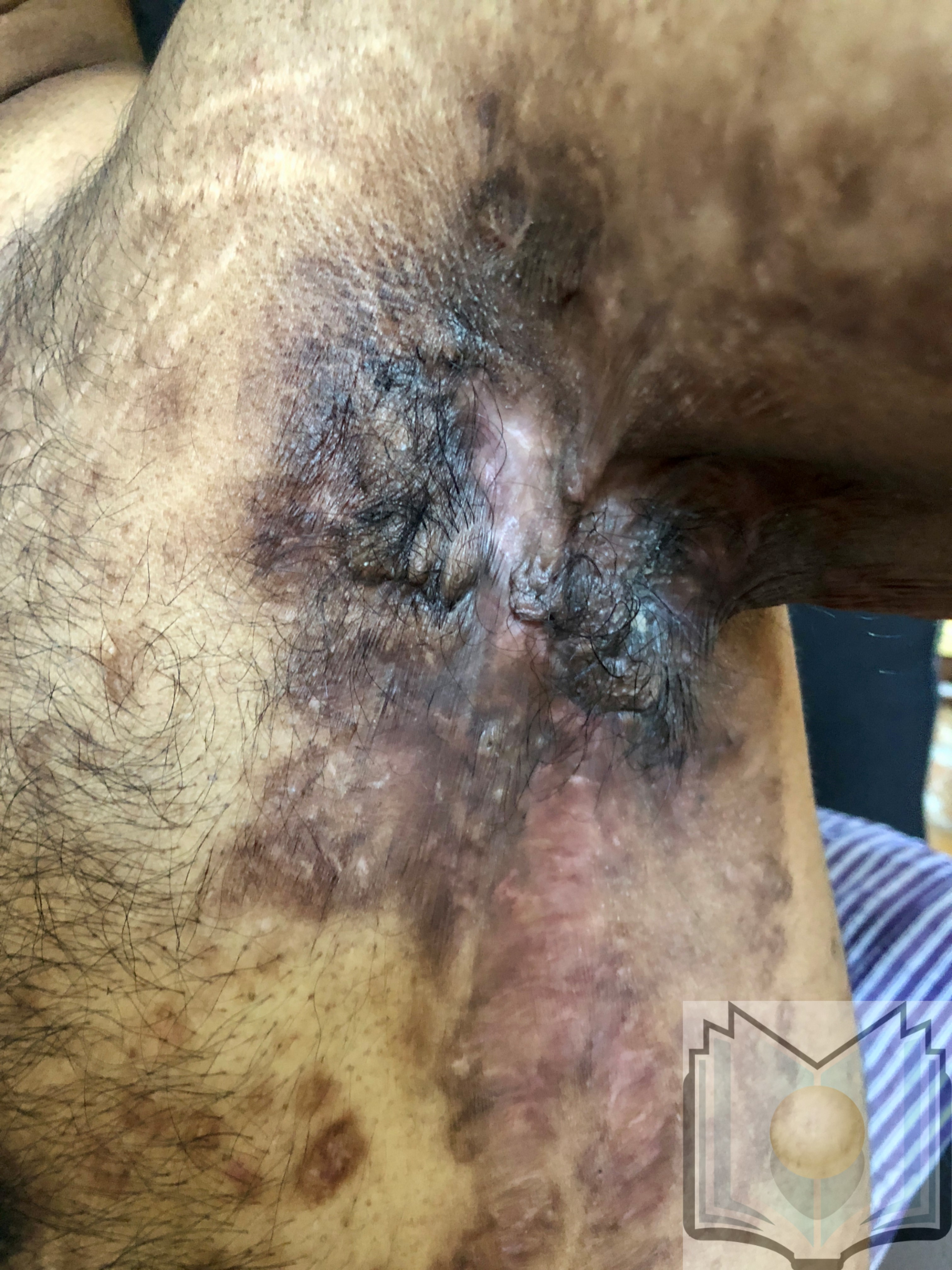
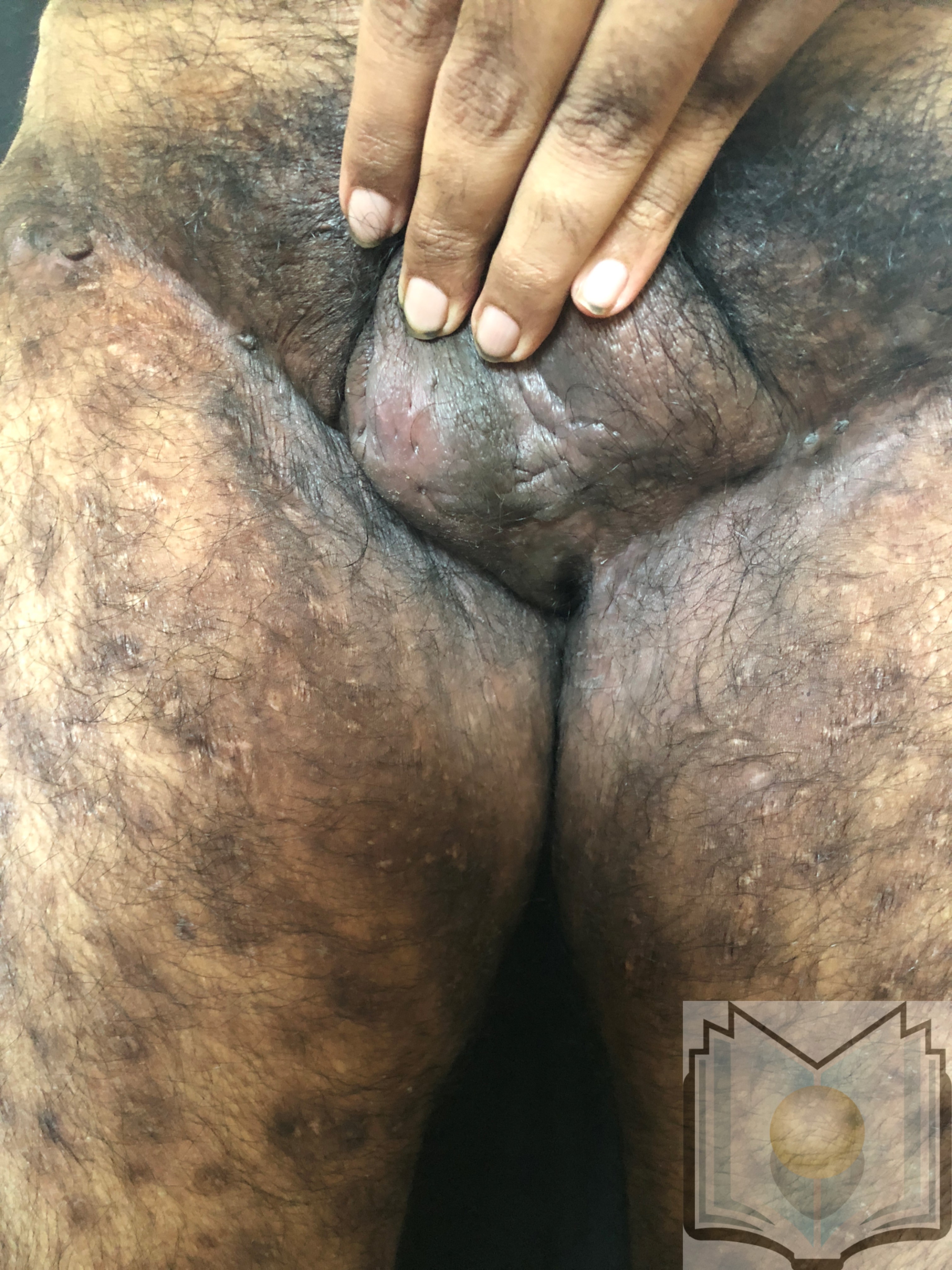
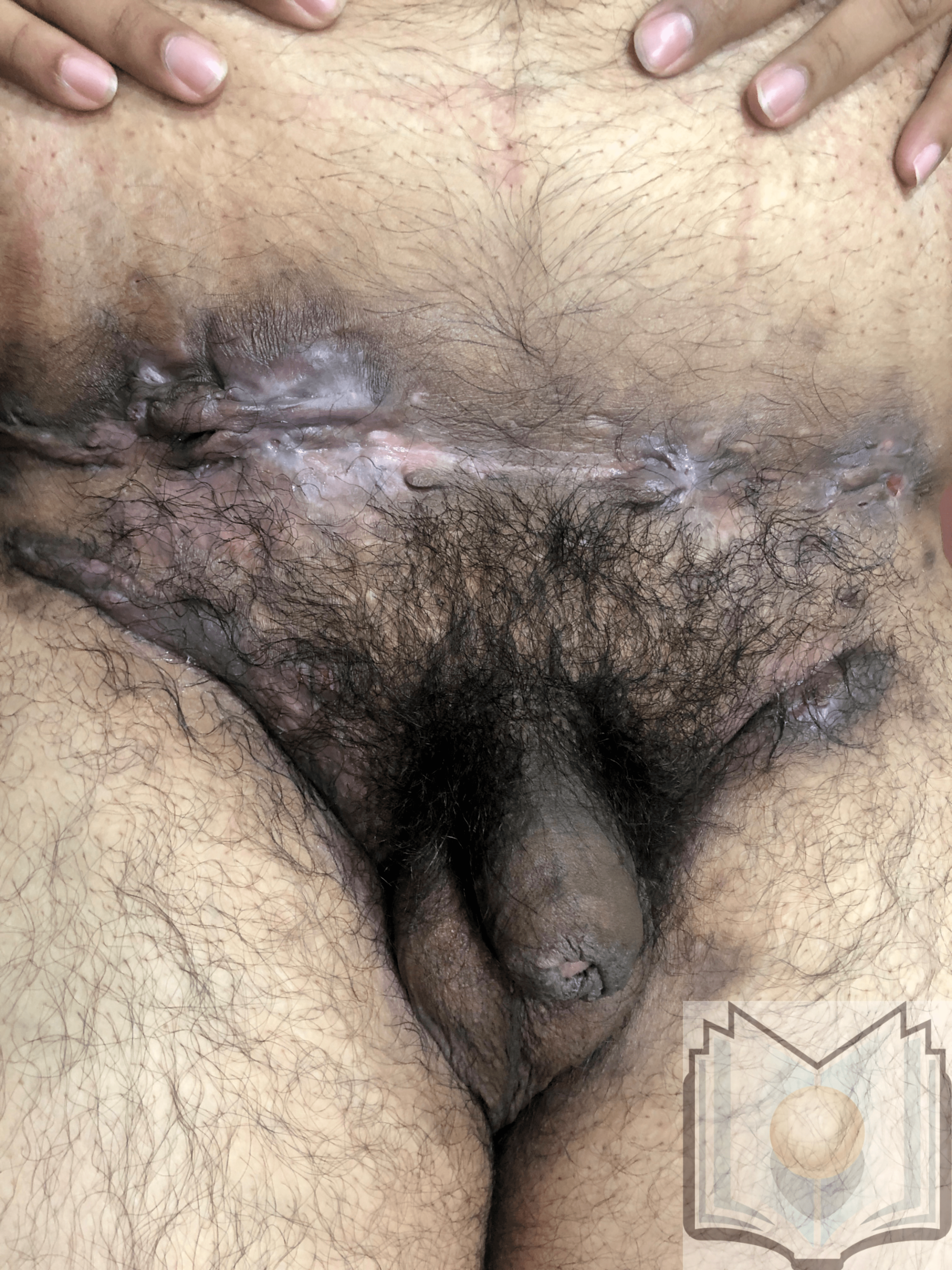
Characteristic primary lesions are deep-seated nodules, usually 0.5 to 2 cm, that last from days to months. They are often mistaken for furuncles or boils; however, a furuncle will respond rapidly to drainage or antibiotics, whereas the nodules of HS are deep and can rupture and track subcutaneously. Multiple recurrent nodules in the same area may lead to the formation of intercommunicating sinus tracts that can ulcerate or drain. Drainage may be purulent and malodorous. Other lesions include open comedones (described as “tombstone” comedones), often double or multiheaded. In advanced stages, thick fibrotic scars and plaques can develop, leading to architectural distortion of the area.[11]
The axilla is the most common location for HS lesions. Other common areas are the inguinal, inner thighs, perianal and perineal, inframammary, buttocks, pubic area, scrotum, vulva, trunk, and, less commonly, the scalp and retro-auricular areas.
Pertinent history includes onset from puberty into young adulthood and a history of recurrent lesions with intermittent improvement or resolution. HS is a chronic condition. Identifying a family history of a similar condition is also helpful in establishing the proper diagnosis.
Once the history and physical exam are complete, the Hurley staging system can be used to classify the case.
- Hurley Stage I: Abscess formation without tracts or scars
- Hurley Stage II: Recurrent abscesses with sinus tracts and scarring; there may be single or widely separated lesions
- Hurley Stage III: Diffuse involvement, multiple interconnected sinus tracts, and abscesses across an entire area, leaving little to no uninvolved skin [12]
HS is one component of the follicular occlusion tetrad, including acne conglobata, dissecting cellulitis of the scalp, and pilonidal sinus. Diagnosis of HS in an individual warrants evaluation for these coexisting diagnoses. Clinicians should also keep in mind the association between metabolic syndrome, inflammatory bowel disease, and spondyloarthropathy and assess for these conditions during the history and physical.
Evaluation
There are no biological or pathological diagnostic tests for HS. Although a biopsy is not required for diagnosis, it is beneficial to rule out squamous cell carcinoma in the presence of severe HS if the diagnosis is uncertain. Bacterial cultures are not beneficial unless a secondary infection or an alternative diagnosis is suspected. Imaging is not typically helpful; however, ultrasound may be a useful tool preoperatively to identify the extent of sinus tracts. Lesions may warrant further imaging, including MRI in severe perianal disease.
Treatment / Management
Overall treatment goals include minimizing pain and drainage of existing lesions, decreasing the frequency of recurrence, and preventing disease progression. Since limited studies compare treatment regimens, most treatment algorithms are based on expert opinion and consensus.
In early uncomplicated diseases, topical antibiotics are the first-line treatment. Topical clindamycin has been proven the most effective.[13] Intralesional corticosteroids can reduce local inflammation, and partial deroofing (punch debridement) of individual lesions can facilitate healing.
Treatment for Hurley Stage II and resistant Hurley Stage I involves oral antibiotics. Antibiotics in the tetracycline family have been shown to be the most effective.[14] If treatment failure persists, combination therapy with oral clindamycin plus rifampin is recommended.[15] Antiandrogenic hormonal therapy can also be helpful, including cyproterone acetate, oral contraceptives, spironolactone, and finasteride.[16] Oral retinoids have shown mixed responsiveness. Isotretinoin is most effective in acne, whereas acitretin appears more effective in HS.[17][18] Systemic steroids are effective for some individuals.
For Hurley stage III and resistant lower stages, tumor necrosis factor-alpha inhibitors are indicated. Adalimumab is the only FDA-approved medication to treat HS.[19][20] At this stage, surgery is often needed and involves a wide excision to include the lesions, tracts, and scars of an entire affected area. A combination of medical treatment and surgical excision is often the preferred approach. Other therapeutic options may include localized laser and pulsed light therapy, which help to disrupt the inflammatory process.[21][22][23]
Pain management is also critical. The pain of HS is both inflammatory and noninflammatory. Sources of pain can include scarring (causing tensile pain), keloids, abscesses, open ulcerations, sinus tracts, frictional pain, lymphedema, anal fissures, and arthritis. Depending on disease severity and type of pain, topical agents (lidocaine and anti-inflammatories), systemic nonsteroidal anti-inflammatories, acetaminophen, atypical anticonvulsants, including gabapentin or pregabalin, and serotonin-norepinephrine reuptake inhibitors may be beneficial. Duloxetine is especially helpful if there is comorbid depression.[24][25]
Regardless of the stage of the disease, treatment should include the management of comorbidities that contribute to the development of or worsening of the disease process. Individuals above ideal weight or who smoke have more severe disease progression, so counseling and help with weight loss and smoking cessation are important components of treatment.[26]
Treatment also involves avoidance of skin trauma. Eliminating tight and synthetic clothing, avoiding harsh products or cleaning tools (loofahs, washcloths, brushes), and avoiding adhesive dressings can be beneficial. Soft dressings with clear petroleum jelly or non-occlusive dressings can be used to prevent further irritation to draining lesions.[27]
A critically important but often overlooked aspect of treatment involves the psychosocial aspect of the disease. Quality of life is diminished in individuals with this condition because of the associated pain, drainage, odor, and sensitive affected areas. Patients may become socially isolated, have employment difficulties because of missed days of work when flares occur, and may have increased sexual or relationship dysfunction. Assurance that this condition is not contagious or the result of poor hygiene can be helpful. Counseling and support groups are often beneficial additions to treatment plans.[28]
Differential Diagnosis
When diagnosing HS, the following conditions should be ruled out:
- Follicular pyoderma (including folliculitis, furuncles, carbuncles)
- Granuloma inguinale
- Noduloulcerative syphilis
- Tuberculous abscess
- Actinomycosis
- Lymphogranuloma venereum
- Acne vulgaris
- Epidermoid, dermoid, pilonidal, or Bartholin cysts
- Crohn disease (particularly with perianal involvement) [29][30][31]
Prognosis
The prognosis of HS is variable. There is no cure for this condition. The prognosis worsens if there is a delay in diagnosis and treatment during the early stages of the disease and if comorbid conditions of smoking and obesity (if present) are not addressed and improved.[32]
Complications
Complications of HS include both physical and psychological conditions.[33] Physically, a recurrence of lesions leading to abscesses, tracts, and scarring can cause chronic pain, limb contractures, and impaired mobility. Lymphatic obstruction can lead to peripheral lymphedema. Long-term effects of chronic inflammation can also occur, including anemia, hyperproteinemia, and amyloidosis, as well as axial and peripheral arthropathy. In rare cases, a superimposed infection can lead to systemic illness of variable severity. Squamous cell carcinoma can occur in the setting of HS, sometimes occurring up to 30 years after diagnosis. There is an associated increase in buccal and hepatocellular cancer based on observational data.
HS can have a psychological impact as well. Combining chronic pain with drainage, odor, and deformity of skin appearance can lead to depression, social isolation, decreased relationship satisfaction, sexual dysfunction, reduced work productivity, and even suicide in extreme cases.[33]
Deterrence and Patient Education
Patient education involves reassurance that the condition is not infectious or caused by poor hygiene. Patients should report lesions as soon as they occur so that proper treatment can be initiated for immediate relief and avoidance of chronic worsening of the condition. Individuals should also be counseled on lifestyle modifications, including maintenance of a healthy weight, smoking cessation, and avoiding skin trauma by wearing looser fitting clothing and avoiding abrasive cleansing and adhesive bandages. Education regarding the risks and benefits of various treatments should be provided, with the most effective treatment for the current disease state offered. Psychosocial education and treatment is critical.
Pearls and Other Issues
- HS is a chronic, inflammatory skin disorder with lesions ranging from deep-seated, painful nodules to abscesses with sinus tract formation to dense fibronodular scarring.
- HS begins with obstruction of hair follicles, followed by rupture and subsequent inflammatory reaction. Infection does not cause HS. It is not contagious and is not the result of poor hygiene.
- The development of HS appears to have both hereditary and environmental influences. The strongest associated external influences are obesity and smoking.
- Treatment options include medications (topical and oral antibiotics, intralesional and oral steroids, hormone therapy, and immune modulators) and surgery ranging from lesion deroofing to wide excision.
- HS can cause significant psychosocial distress because of pain, drainage, odor, and location of lesions.
- The best prognosis involves early recognition and aggressive treatment at the early stages of the disease. Treatment includes psychosocial support.
Enhancing Healthcare Team Outcomes
HS is a complex condition with numerous comorbidities. An interprofessional approach is most effective in the management of this disease.[34]
Although HS is considered a dermatologic condition, individuals do not always present to the dermatology office with symptoms. Often, family and internal medicine clinicians, pediatricians, and obstetricians/gynecologists are the individual's first contact with the healthcare system. It is critical for primary care providers to recognize HS based on the morphology, location, and chronicity of lesions and begin the correct treatment early in the disease process. Depending on the severity of the disease and the training of the primary providers, they may treat independently or refer to specialists, including dermatologists, surgeons, wound care, and pain management.
In addition to clinicians, nurses, pharmacists, and other allied healthcare providers may also form part of the interprofessional team treating HS. Nurses can counsel patients, assist with the history and examination, and serve as a contact point for the clinicians for patients and other team members. Pharmacists will verify drug dosing, check for interactions, and counsel patients on proper administration. Since there is a significant psychosocial impact associated with this disease, the treatment team should also include providers of mental health support.
If not provided at the primary care level, psychiatrists, psychologists, or counselors should be involved as needed. All interprofessional team members are responsible for accurately documenting their observations and interventions in the patient's health record so all team members have the same data from which to operate. Open communication is essential if anyone on the care team notes an issue with the patient's case. This interprofessional paradigm will drive optimal patient outcomes.