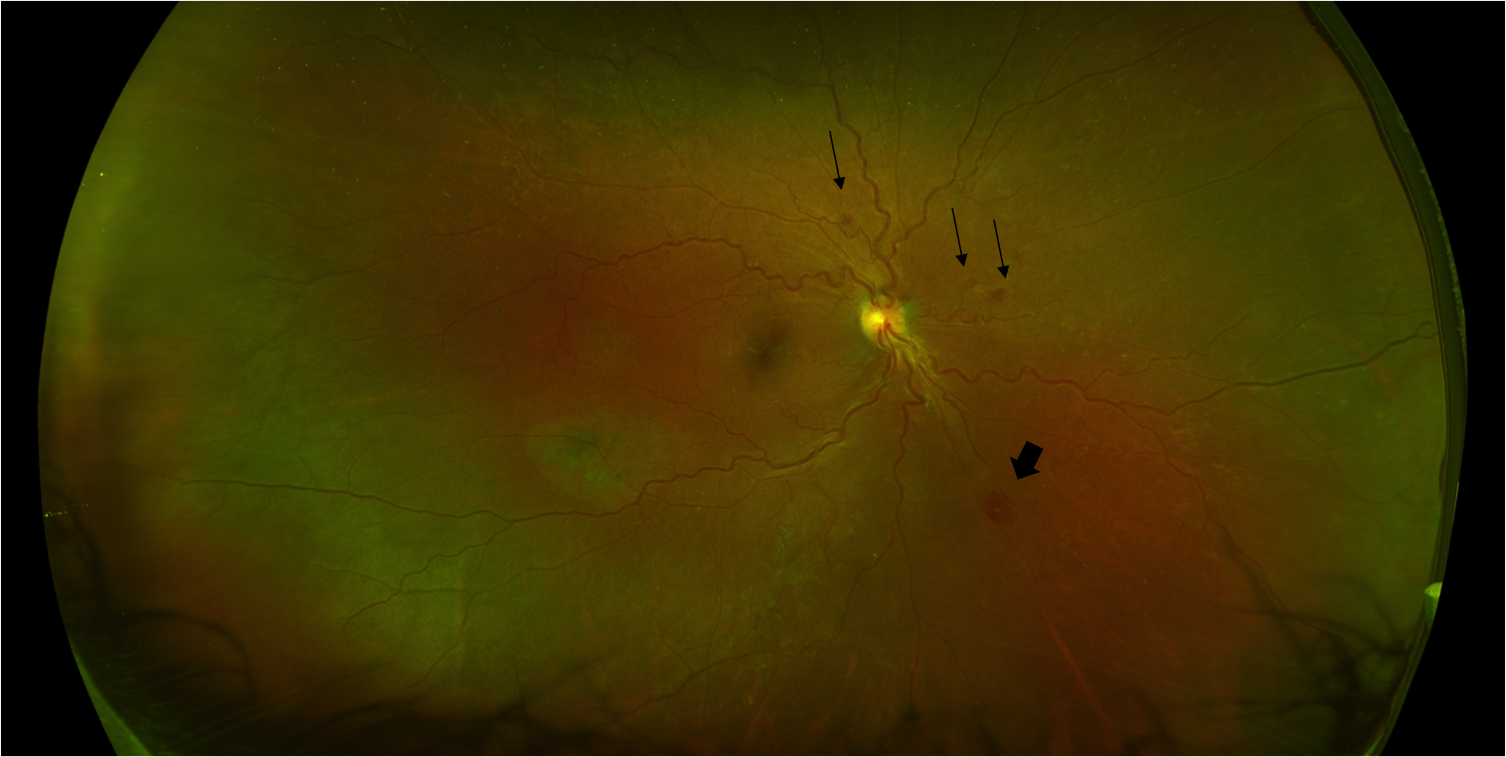
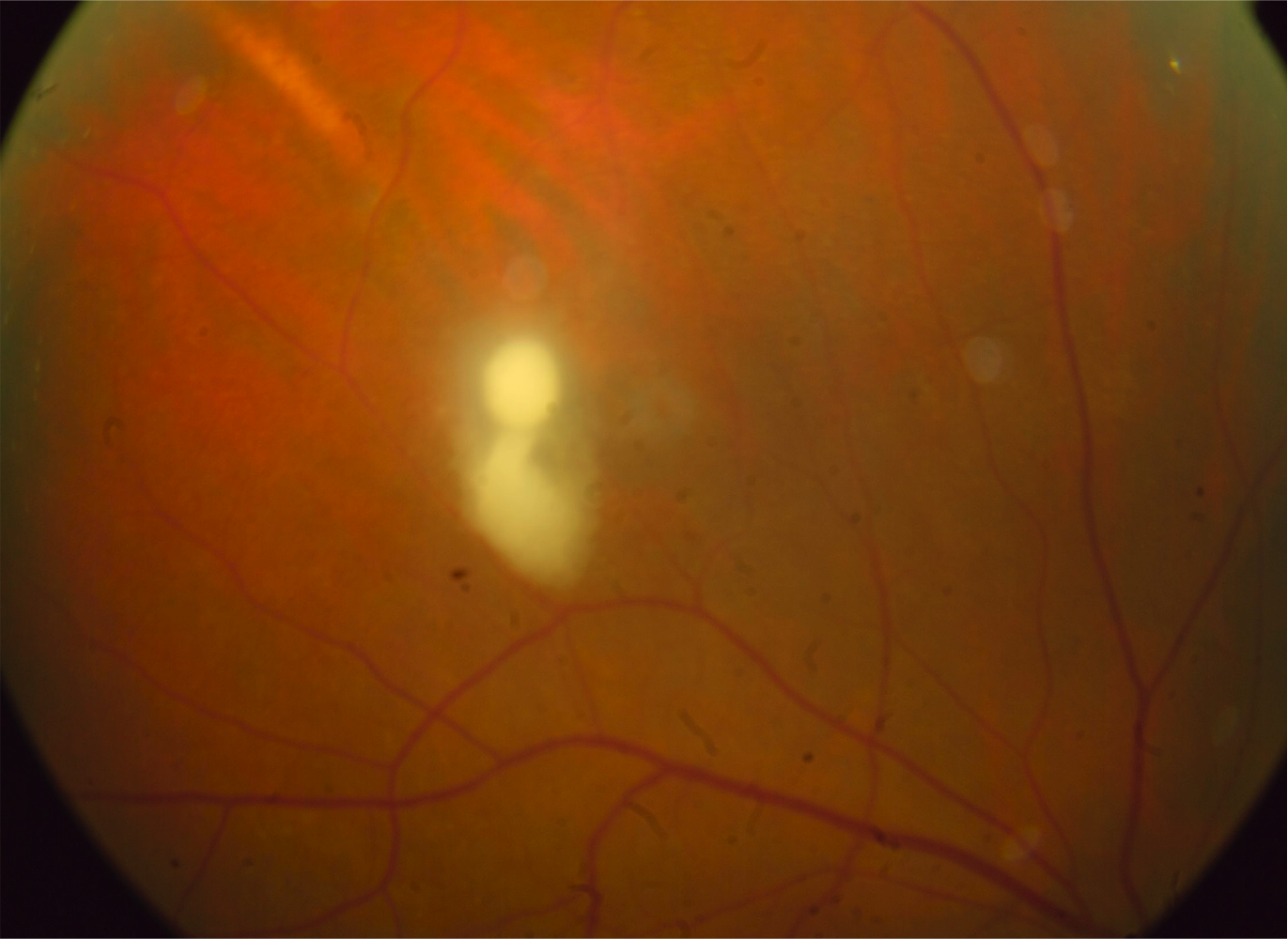
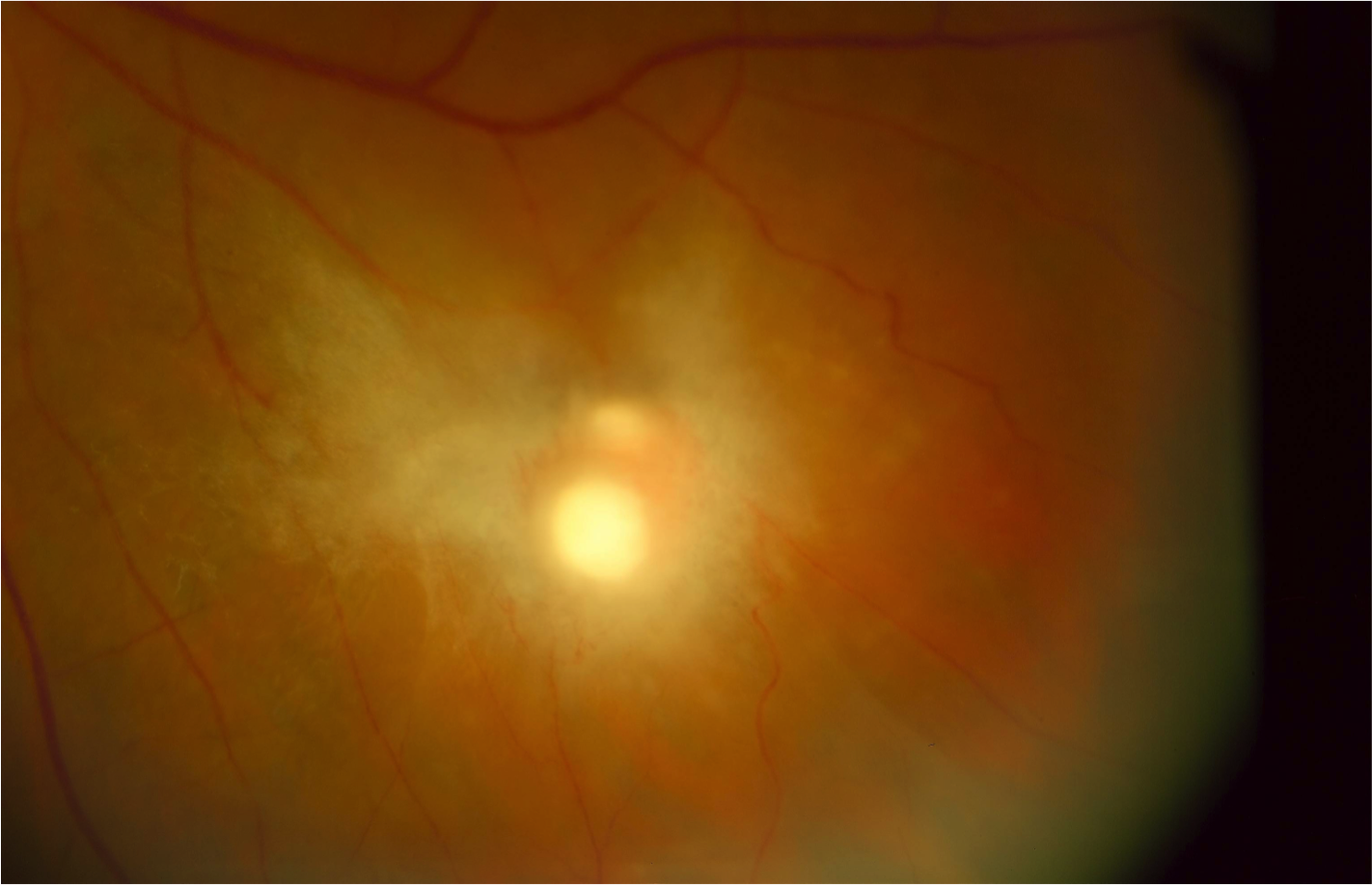
[1]
Durand ML. Bacterial and Fungal Endophthalmitis. Clinical microbiology reviews. 2017 Jul:30(3):597-613. doi: 10.1128/CMR.00113-16. Epub
[PubMed PMID: 28356323]
[2]
Vaziri K, Schwartz SG, Kishor K, Flynn HW Jr. Endophthalmitis: state of the art. Clinical ophthalmology (Auckland, N.Z.). 2015:9():95-108. doi: 10.2147/OPTH.S76406. Epub 2015 Jan 8
[PubMed PMID: 25609911]
[3]
Gupta A, Gupta V, Gupta A, Dogra MR, Pandav SS, Ray P, Chakraborty A. Spectrum and clinical profile of post cataract surgery endophthalmitis in north India. Indian journal of ophthalmology. 2003 Jun:51(2):139-45
[PubMed PMID: 12831144]
[4]
Wykoff CC, Flynn HW Jr, Miller D, Scott IU, Alfonso EC. Exogenous fungal endophthalmitis: microbiology and clinical outcomes. Ophthalmology. 2008 Sep:115(9):1501-7, 1507.e1-2. doi: 10.1016/j.ophtha.2008.02.027. Epub 2008 May 16
[PubMed PMID: 18486220]
Level 2 (mid-level) evidence
[5]
Chen JY, Jones MN, Srinivasan S, Neal TJ, Armitage WJ, Kaye SB, NHSBT Ocular Tissue Advisory Group and Contributing Ophthalmologists (OTAG Audit Study 18). Endophthalmitis after penetrating keratoplasty. Ophthalmology. 2015 Jan:122(1):25-30. doi: 10.1016/j.ophtha.2014.07.038. Epub 2014 Sep 26
[PubMed PMID: 25264028]
[6]
Kernt M, Kampik A. Endophthalmitis: Pathogenesis, clinical presentation, management, and perspectives. Clinical ophthalmology (Auckland, N.Z.). 2010 Mar 24:4():121-35
[PubMed PMID: 20390032]
Level 3 (low-level) evidence
[7]
Long C, Liu B, Xu C, Jing Y, Yuan Z, Lin X. Causative organisms of post-traumatic endophthalmitis: a 20-year retrospective study. BMC ophthalmology. 2014 Mar 25:14():34. doi: 10.1186/1471-2415-14-34. Epub 2014 Mar 25
[PubMed PMID: 24661397]
Level 2 (mid-level) evidence
[8]
Henry CR, Flynn HW Jr, Miller D, Forster RK, Alfonso EC. Infectious keratitis progressing to endophthalmitis: a 15-year study of microbiology, associated factors, and clinical outcomes. Ophthalmology. 2012 Dec:119(12):2443-9. doi: 10.1016/j.ophtha.2012.06.030. Epub 2012 Aug 1
[PubMed PMID: 22858123]
Level 2 (mid-level) evidence
[9]
Feman SS, Nichols JC, Chung SM, Theobald TA. Endophthalmitis in patients with disseminated fungal disease. Transactions of the American Ophthalmological Society. 2002:100():67-70; discussion 70-1
[PubMed PMID: 12545679]
[10]
Shin SU, Yu YH, Kim SS, Oh TH, Kim SE, Kim UJ, Kang SJ, Jang HC, Park KH, Jung SI. Clinical characteristics and risk factors for complications of candidaemia in adults: Focus on endophthalmitis, endocarditis, and osteoarticular infections. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2020 Apr:93():126-132. doi: 10.1016/j.ijid.2020.01.049. Epub 2020 Jan 30
[PubMed PMID: 32007642]
[11]
Dozier CC, Tarantola RM, Jiramongkolchai K, Donahue SP. Fungal eye disease at a tertiary care center: the utility of routine inpatient consultation. Ophthalmology. 2011 Aug:118(8):1671-6. doi: 10.1016/j.ophtha.2011.01.038. Epub 2011 May 6
[PubMed PMID: 21550121]
[12]
Vilela RC, Vilela L, Vilela P, Vilela R, Motta R, Pôssa AP, de Almeida C, Mendoza L. Etiological agents of fungal endophthalmitis: diagnosis and management. International ophthalmology. 2014 Jun:34(3):707-21. doi: 10.1007/s10792-013-9854-z. Epub 2013 Oct 1
[PubMed PMID: 24081913]
[13]
Griffin JR, Pettit TH, Fishman LS, Foos RY. Blood-borne Candida endophthalmitis. A clinical and pathologic study of 21 cases. Archives of ophthalmology (Chicago, Ill. : 1960). 1973 Jun:89(6):450-6
[PubMed PMID: 4706441]
Level 3 (low-level) evidence
[14]
Donahue SP, Greven CM, Zuravleff JJ, Eller AW, Nguyen MH, Peacock JE Jr, Wagener MW, Yu VL. Intraocular candidiasis in patients with candidemia. Clinical implications derived from a prospective multicenter study. Ophthalmology. 1994 Jul:101(7):1302-9
[PubMed PMID: 8035995]
Level 2 (mid-level) evidence
[15]
McDonnell PJ, McDonnell JM, Brown RH, Green WR. Ocular involvement in patients with fungal infections. Ophthalmology. 1985 May:92(5):706-9
[PubMed PMID: 3874382]
[16]
Brooks RG. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Archives of internal medicine. 1989 Oct:149(10):2226-8
[PubMed PMID: 2802888]
[17]
Bross J, Talbot GH, Maislin G, Hurwitz S, Strom BL. Risk factors for nosocomial candidemia: a case-control study in adults without leukemia. The American journal of medicine. 1989 Dec:87(6):614-20
[PubMed PMID: 2589396]
Level 2 (mid-level) evidence
[18]
Krishna R, Amuh D, Lowder CY, Gordon SM, Adal KA, Hall G. Should all patients with candidaemia have an ophthalmic examination to rule out ocular candidiasis? Eye (London, England). 2000 Feb:14 ( Pt 1)():30-4
[PubMed PMID: 10755096]
[19]
Shah CP, McKey J, Spirn MJ, Maguire J. Ocular candidiasis: a review. The British journal of ophthalmology. 2008 Apr:92(4):466-8. doi: 10.1136/bjo.2007.133405. Epub
[PubMed PMID: 18369061]
[20]
Kannangara S, Shindler D, Kunimoto DY, Sell B, DeSimone JA. Candidemia complicated by endophthalmitis: a prospective analysis. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 2007 Nov:26(11):839-41
[PubMed PMID: 17668252]
[21]
Sallam A, Lynn W, McCluskey P, Lightman S. Endogenous Candida endophthalmitis. Expert review of anti-infective therapy. 2006 Aug:4(4):675-85
[PubMed PMID: 17009945]
[22]
Klotz SA, Penn CC, Negvesky GJ, Butrus SI. Fungal and parasitic infections of the eye. Clinical microbiology reviews. 2000 Oct:13(4):662-85
[PubMed PMID: 11023963]
[23]
Sadiq MA, Hassan M, Agarwal A, Sarwar S, Toufeeq S, Soliman MK, Hanout M, Sepah YJ, Do DV, Nguyen QD. Endogenous endophthalmitis: diagnosis, management, and prognosis. Journal of ophthalmic inflammation and infection. 2015 Dec:5(1):32. doi: 10.1186/s12348-015-0063-y. Epub 2015 Nov 3
[PubMed PMID: 26525563]
[24]
Okada AA, Johnson RP, Liles WC, D'Amico DJ, Baker AS. Endogenous bacterial endophthalmitis. Report of a ten-year retrospective study. Ophthalmology. 1994 May:101(5):832-8
[PubMed PMID: 8190467]
Level 2 (mid-level) evidence
[25]
Wu ZH, Chan RP, Luk FO, Liu DT, Chan CK, Lam DS, Lai TY. Review of Clinical Features, Microbiological Spectrum, and Treatment Outcomes of Endogenous Endophthalmitis over an 8-Year Period. Journal of ophthalmology. 2012:2012():265078. doi: 10.1155/2012/265078. Epub 2012 Feb 23
[PubMed PMID: 23533699]
[26]
Mamandhar A, Bajracharya L. Endogenous Aspergillus endophthalmitis in a healthy individual. Nepalese journal of ophthalmology : a biannual peer-reviewed academic journal of the Nepal Ophthalmic Society : NEPJOPH. 2012 Jan-Jun:4(1):179-83. doi: 10.3126/nepjoph.v4i1.5873. Epub
[PubMed PMID: 22344019]
[27]
Logan S, Rajan M, Graham E, Johnson E, Klein J. A case of aspergillus endophthalmitis in an immuncompetent woman: intra-ocular penetration of oral voriconazole: a case report. Cases journal. 2010 Jan 18:3():31. doi: 10.1186/1757-1626-3-31. Epub 2010 Jan 18
[PubMed PMID: 20205770]
Level 3 (low-level) evidence
[28]
Agarwal M, Biswas J, Mathur U, Sijwali MS, Singh AK. Aspergillus iris granuloma in a young male: a case report with review of literature. Indian journal of ophthalmology. 2007 Jan-Feb:55(1):73-4
[PubMed PMID: 17189896]
Level 3 (low-level) evidence
[29]
Lee JH, Kim JS, Park YH. Diagnosis and treatment of postpartum Candida endophthalmitis. The journal of obstetrics and gynaecology research. 2012 Sep:38(9):1220-2. doi: 10.1111/j.1447-0756.2012.01854.x. Epub 2012 May 8
[PubMed PMID: 22563724]
[30]
Shankar K, Gyanendra L, Hari S, Narayan SD. Culture proven endogenous bacterial endophthalmitis in apparently healthy individuals. Ocular immunology and inflammation. 2009 Nov-Dec:17(6):396-9. doi: 10.3109/09273940903216891. Epub
[PubMed PMID: 20001259]
[31]
Tsirouki T, Steffen J, Dastiridou A, Praidou A, Androudi S. Endophthalmitis in HIV Infection. Ocular immunology and inflammation. 2020 Oct 2:28(7):1060-1065. doi: 10.1080/09273948.2019.1699580. Epub 2020 Jan 16
[PubMed PMID: 31944150]
[32]
Edwards JE Jr, Montgomerie JZ, Foos RY, Shaw VK, Guze LB. Experimental hematogenous endophthalmitis caused by Candida albicans. The Journal of infectious diseases. 1975 Jun:131(6):649-57
[PubMed PMID: 48529]
[33]
Moshfeghi AA, Charalel RA, Hernandez-Boussard T, Morton JM, Moshfeghi DM. Declining incidence of neonatal endophthalmitis in the United States. American journal of ophthalmology. 2011 Jan:151(1):59-65.e1. doi: 10.1016/j.ajo.2010.07.008. Epub
[PubMed PMID: 20970776]
[34]
Aziz HA, Berrocal AM, Sisk RA, Hartley K, Diaz-Barbosa M, Johnson RA, Hess D, Dubovy SR, Murray TG, Flynn HW Jr. Intraocular infections in the neonatal intensive care unit. Clinical ophthalmology (Auckland, N.Z.). 2012:6():733-7. doi: 10.2147/OPTH.S26362. Epub 2012 May 14
[PubMed PMID: 22654500]
[35]
Basu S, Kumar A, Kapoor K, Bagri NK, Chandra A. Neonatal endogenous endophthalmitis: a report of six cases. Pediatrics. 2013 Apr:131(4):e1292-7. doi: 10.1542/peds.2011-3391. Epub 2013 Mar 11
[PubMed PMID: 23478867]
Level 3 (low-level) evidence
[36]
Stone J, Chan-Ling T, Pe'er J, Itin A, Gnessin H, Keshet E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Investigative ophthalmology & visual science. 1996 Feb:37(2):290-9
[PubMed PMID: 8603833]
[37]
Kaburaki T, Sato S, Kawashima H, Sakurai M, Numaga J, Fujino Y, Araie M. A hypopyon is a sign of post-trabeculectomy endophthalmitis or not? Eye (London, England). 2005 Jun:19(6):692-3
[PubMed PMID: 15184940]
[38]
Sheyman AT, Cohen BZ, Friedman AH, Ackert JM. An outbreak of fungal endophthalmitis after intravitreal injection of compounded combined bevacizumab and triamcinolone. JAMA ophthalmology. 2013 Jul:131(7):864-9. doi: 10.1001/jamaophthalmol.2013.88. Epub
[PubMed PMID: 23640384]
[39]
Rao NA, Hidayat A. A comparative clinicopathologic study of endogenous mycotic endophthalmitis: variations in clinical and histopathologic changes in candidiasis compared to aspergillosis. Transactions of the American Ophthalmological Society. 2000:98():183-93; discussion 193-4
[PubMed PMID: 11190022]
Level 2 (mid-level) evidence
[40]
Riddell Iv J, McNeil SA, Johnson TM, Bradley SF, Kazanjian PH, Kauffman CA. Endogenous Aspergillus endophthalmitis: report of 3 cases and review of the literature. Medicine. 2002 Jul:81(4):311-20
[PubMed PMID: 12169886]
Level 3 (low-level) evidence
[41]
Davis JL. Diagnostic dilemmas in retinitis and endophthalmitis. Eye (London, England). 2012 Feb:26(2):194-201. doi: 10.1038/eye.2011.299. Epub 2011 Nov 25
[PubMed PMID: 22116459]
[42]
Tanaka M, Kobayashi Y, Takebayashi H, Kiyokawa M, Qiu H. Analysis of predisposing clinical and laboratory findings for the development of endogenous fungal endophthalmitis. A retrospective 12-year study of 79 eyes of 46 patients. Retina (Philadelphia, Pa.). 2001:21(3):203-9
[PubMed PMID: 11421007]
Level 2 (mid-level) evidence
[43]
Lingappan A, Wykoff CC, Albini TA, Miller D, Pathengay A, Davis JL, Flynn HW Jr. Endogenous fungal endophthalmitis: causative organisms, management strategies, and visual acuity outcomes. American journal of ophthalmology. 2012 Jan:153(1):162-6.e1. doi: 10.1016/j.ajo.2011.06.020. Epub 2011 Sep 13
[PubMed PMID: 21917234]
[44]
Denning DW, Evans EG, Kibbler CC, Richardson MD, Roberts MM, Rogers TR, Warnock DW, Warren RE. Guidelines for the investigation of invasive fungal infections in haematological malignancy and solid organ transplantation. British Society for Medical Mycology. European journal of clinical microbiology & infectious diseases : official publication of the European Society of Clinical Microbiology. 1997 Jun:16(6):424-36
[PubMed PMID: 9248745]
[45]
Sugita S, Kamoi K, Ogawa M, Watanabe K, Shimizu N, Mochizuki M. Detection of Candida and Aspergillus species DNA using broad-range real-time PCR for fungal endophthalmitis. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2012 Mar:250(3):391-8. doi: 10.1007/s00417-011-1819-1. Epub 2011 Sep 27
[PubMed PMID: 21947326]
[46]
Sandhu HS, Hajrasouliha A, Kaplan HJ, Wang W. Diagnostic Utility of Quantitative Polymerase Chain Reaction versus Culture in Endophthalmitis and Uveitis. Ocular immunology and inflammation. 2019:27(4):578-582. doi: 10.1080/09273948.2018.1431291. Epub 2018 Feb 22
[PubMed PMID: 29470930]
[47]
Sowmya P, Madhavan HN. Diagnostic utility of polymerase chain reaction on intraocular specimens to establish the etiology of infectious endophthalmitis. European journal of ophthalmology. 2009 Sep-Oct:19(5):812-7
[PubMed PMID: 19787602]
[48]
Kharel Sitaula R, Janani MK, Madhavan HN, Biswas J. Outcome of polymerase chain reaction (PCR) analysis in 100 suspected cases of infectious uveitis. Journal of ophthalmic inflammation and infection. 2018 Jan 10:8(1):2. doi: 10.1186/s12348-017-0144-1. Epub 2018 Jan 10
[PubMed PMID: 29322275]
Level 3 (low-level) evidence
[49]
Chen L, Tao Y, Hu X. Utility of Intraocular Fluid β-D-glucan Testing in Fungal Endophthalmitis: A Series of 5 Cases. The American journal of case reports. 2020 Mar 23:21():e921188. doi: 10.12659/AJCR.921188. Epub 2020 Mar 23
[PubMed PMID: 32201431]
Level 3 (low-level) evidence
[50]
Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2016 Feb 15:62(4):e1-50. doi: 10.1093/cid/civ933. Epub 2015 Dec 16
[PubMed PMID: 26679628]
Level 1 (high-level) evidence
[51]
Sallam A, Taylor SR, Khan A, McCluskey P, Lynn WA, Manku K, Pacheco PA, Lightman S. Factors determining visual outcome in endogenous Candida endophthalmitis. Retina (Philadelphia, Pa.). 2012 Jun:32(6):1129-34. doi: 10.1097/IAE.0b013e31822d3a34. Epub
[PubMed PMID: 22298012]
[52]
Silva RA, Sridhar J, Miller D, Wykoff CC, Flynn HW Jr. Exogenous fungal endophthalmitis: an analysis of isolates and susceptibilities to antifungal agents over a 20-year period (1990-2010). American journal of ophthalmology. 2015 Feb:159(2):257-64.e1. doi: 10.1016/j.ajo.2014.10.027. Epub 2014 Nov 1
[PubMed PMID: 25449001]
[53]
Sen P, Gopal L, Sen PR. Intravitreal voriconazole for drug-resistant fungal endophthalmitis: case series. Retina (Philadelphia, Pa.). 2006 Oct:26(8):935-9
[PubMed PMID: 17031296]
Level 2 (mid-level) evidence
[54]
Hariprasad SM, Mieler WF, Lin TK, Sponsel WE, Graybill JR. Voriconazole in the treatment of fungal eye infections: a review of current literature. The British journal of ophthalmology. 2008 Jul:92(7):871-8. doi: 10.1136/bjo.2007.136515. Epub
[PubMed PMID: 18577634]
[55]
Zhang H, Liu Z. Endogenous endophthalmitis: a 10-year review of culture-positive cases in northern China. Ocular immunology and inflammation. 2010 Apr:18(2):133-8. doi: 10.3109/09273940903494717. Epub
[PubMed PMID: 20370344]
Level 3 (low-level) evidence
[56]
Sridhar J, Flynn HW Jr, Kuriyan AE, Miller D, Albini T. Endogenous fungal endophthalmitis: risk factors, clinical features, and treatment outcomes in mold and yeast infections. Journal of ophthalmic inflammation and infection. 2013 Sep 20:3(1):60. doi: 10.1186/1869-5760-3-60. Epub 2013 Sep 20
[PubMed PMID: 24053550]
[57]
Duan F, Yang Y, Yuan Z, Zheng Y, Cheng Z, Lin X. Clinical Features and Visual Acuity Outcomes in Culture-Positive Endogenous Fungal Endophthalmitis in Southern China. Journal of ophthalmology. 2017:2017():3483497. doi: 10.1155/2017/3483497. Epub 2017 Aug 13
[PubMed PMID: 28884023]
[58]
Behera UC, Budhwani M, Das T, Basu S, Padhi TR, Barik MR, Sharma S. ROLE OF EARLY VITRECTOMY IN THE TREATMENT OF FUNGAL ENDOPHTHALMITIS. Retina (Philadelphia, Pa.). 2018 Jul:38(7):1385-1392. doi: 10.1097/IAE.0000000000001727. Epub
[PubMed PMID: 28541964]
[59]
Binder MI, Chua J, Kaiser PK, Procop GW, Isada CM. Endogenous endophthalmitis: an 18-year review of culture-positive cases at a tertiary care center. Medicine. 2003 Mar:82(2):97-105
[PubMed PMID: 12640186]
Level 3 (low-level) evidence
[60]
Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Jun 15:48(12):1695-703. doi: 10.1086/599039. Epub
[PubMed PMID: 19441981]
[61]
Schiedler V, Scott IU, Flynn HW Jr, Davis JL, Benz MS, Miller D. Culture-proven endogenous endophthalmitis: clinical features and visual acuity outcomes. American journal of ophthalmology. 2004 Apr:137(4):725-31
[PubMed PMID: 15059712]