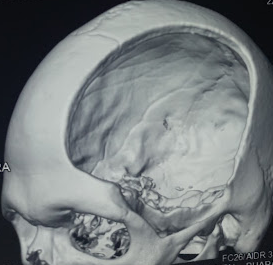
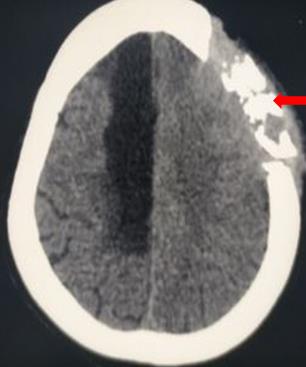
[3]
Bhaskar IP,Zaw NN,Zheng M,Lee GY, Bone flap storage following craniectomy: a survey of practices in major Australian neurosurgical centres. ANZ journal of surgery. 2011 Mar;
[PubMed PMID: 21342384]
Level 3 (low-level) evidence
[4]
Schizodimos T,Soulountsi V,Iasonidou C,Kapravelos N, An overview of management of intracranial hypertension in the intensive care unit. Journal of anesthesia. 2020 May 21;
[PubMed PMID: 32440802]
Level 3 (low-level) evidence
[5]
Sahuquillo J,Dennis JA, Decompressive craniectomy for the treatment of high intracranial pressure in closed traumatic brain injury. The Cochrane database of systematic reviews. 2019 Dec 31;
[PubMed PMID: 31887790]
Level 1 (high-level) evidence
[6]
Alkhaibary A,Alharbi A,Alnefaie N,Aloraidi A,Khairy S, Cranioplasty: A Comprehensive Review of the History, Materials, Surgical Aspects and Complications. World neurosurgery. 2020 May 5;
[PubMed PMID: 32387405]
[8]
Buchfelder M, From trephination to tailored resection: neurosurgery in Germany before World War II. Neurosurgery. 2005 Mar;
[PubMed PMID: 15730586]
[9]
Andrushko VA,Verano JW, Prehistoric trepanation in the Cuzco region of Peru: a view into an ancient Andean practice. American journal of physical anthropology. 2008 Sep;
[PubMed PMID: 18386793]
[12]
Rao D,Le RT,Fiester P,Patel J,Rahmathulla G, An Illustrative Review of Common Modern Craniotomies. Journal of clinical imaging science. 2020;
[PubMed PMID: 33408956]
[13]
Sperati G, Craniotomy through the ages. Acta otorhinolaryngologica Italica : organo ufficiale della Societa italiana di otorinolaringologia e chirurgia cervico-facciale. 2007 Jun;
[PubMed PMID: 17883195]
[14]
Yasargil MG,Antic J,Laciga R,Jain KK,Hodosh RM,Smith RD, Microsurgical pterional approach to aneurysms of the basilar bifurcation. Surgical neurology. 1976 Aug;
[PubMed PMID: 951657]
[15]
Yaşargil MG,Reichman MV,Kubik S, Preservation of the frontotemporal branch of the facial nerve using the interfascial temporalis flap for pterional craniotomy. Technical article. Journal of neurosurgery. 1987 Sep;
[PubMed PMID: 3612281]
[16]
Hendricks BK,Cohen-Gadol AA, The Extended Pterional Craniotomy: A Contemporary and Balanced Approach. Operative neurosurgery (Hagerstown, Md.). 2020 Feb 1;
[PubMed PMID: 31172173]
[17]
Choque-Velasquez J,Hernesniemi J, One burr-hole craniotomy: Lateral supraorbital approach in Helsinki Neurosurgery. Surgical neurology international. 2018;
[PubMed PMID: 30159200]
[18]
Choque-Velasquez J,Hernesniemi J, One burr-hole craniotomy: Subtemporal approach in helsinki neurosurgery. Surgical neurology international. 2018;
[PubMed PMID: 30186665]
[19]
Zieliński G,Sajjad EA,Robak Ł,Koziarski A, Subtemporal Approach for Gross Total Resection of Retrochiasmatic Craniopharyngiomas: Our Experience on 30 Cases. World neurosurgery. 2018 Jan;
[PubMed PMID: 28987834]
Level 3 (low-level) evidence
[20]
Zhou C,Evins AI,Boschi A,Tang Y,Li S,Przepiorka L,Sadhwani S,Stieg PE,Xu T,Bernardo A, Preoperative identification of the initial burr hole site in retrosigmoid craniotomies: A teaching and technical note. The international journal of medical robotics computer assisted surgery : MRCAS. 2019 Jun;
[PubMed PMID: 30721556]
[21]
Stachniak JB,Layon AJ,Day AL,Gallagher TJ, Craniotomy for intracranial aneurysm and subarachnoid hemorrhage. Is course, cost, or outcome affected by age? Stroke. 1996 Feb;
[PubMed PMID: 8571423]
[22]
Legnani FG,Saladino A,Casali C,Vetrano IG,Varisco M,Mattei L,Prada F,Perin A,Mangraviti A,Solero CL,DiMeco F, Craniotomy vs. craniectomy for posterior fossa tumors: a prospective study to evaluate complications after surgery. Acta neurochirurgica. 2013 Dec;
[PubMed PMID: 24078114]
[23]
Hamasaki T,Morioka M,Nakamura H,Yano S,Hirai T,Kuratsu J, A 3-dimensional computed tomographic procedure for planning retrosigmoid craniotomy. Neurosurgery. 2009 May;
[PubMed PMID: 19404104]
[24]
Broggi G,Broggi M,Ferroli P,Franzini A, Surgical technique for trigeminal microvascular decompression. Acta neurochirurgica. 2012 Jun;
[PubMed PMID: 22531963]
[25]
Rauen K,Reichelt L,Probst P,Schäpers B,Müller F,Jahn K,Plesnila N, Decompressive Craniectomy Is Associated With Good Quality of Life Up to 10 Years After Rehabilitation From Traumatic Brain Injury. Critical care medicine. 2020 May 18;
[PubMed PMID: 32433123]
Level 2 (mid-level) evidence
[26]
Alvis-Miranda H,Castellar-Leones SM,Moscote-Salazar LR, Decompressive Craniectomy and Traumatic Brain Injury: A Review. Bulletin of emergency and trauma. 2013 Apr;
[PubMed PMID: 27162826]
[28]
Duckworth EA,Vale FL, Trephine epilepsy surgery: the inferior temporal gyrus approach. Neurosurgery. 2008 Jul;
[PubMed PMID: 18728594]
[29]
Yang PF,Zhang HJ,Pei JS,Lin Q,Mei Z,Chen ZQ,Jia YZ,Zhong ZH,Zheng ZY, Keyhole epilepsy surgery: corticoamygdalohippocampectomy for mesial temporal sclerosis. Neurosurgical review. 2016 Jan;
[PubMed PMID: 26277790]
[31]
Akai T,Shiraga S,Sasagawa Y,Iizuka H,Yamashita M,Kawakami S, Troubleshooting distraction osteogenesis for craniosynostosis. Pediatric neurosurgery. 2013;
[PubMed PMID: 25500456]
[32]
Alford J,Derderian CA,Smartt JM Jr, Surgical Treatment of Nonsyndromic Unicoronal Craniosynostosis. The Journal of craniofacial surgery. 2018 Jul;
[PubMed PMID: 29570518]
[33]
Ciporen J,Lucke-Wold B,Gillham H,Cua D,Kim J,Akins P, Paramedian Forehead Flap for Repair of Refractory High-Flow Anterior Skull Base CSF Leak. Turkish neurosurgery. 2017 Jul 11;
[PubMed PMID: 28944942]
[34]
Sivanaser V,Manninen P, Preoperative assessment of adult patients for intracranial surgery. Anesthesiology research and practice. 2010;
[PubMed PMID: 20700431]
[36]
Donovan DJ,Moquin RR,Ecklund JM, Cranial burr holes and emergency craniotomy: review of indications and technique. Military medicine. 2006 Jan;
[PubMed PMID: 16532867]
[37]
Greuter L,Ullmann M,Mariani L,Guzman R,Soleman J, Effect of preoperative antiplatelet or anticoagulation therapy on hemorrhagic complications in patients with traumatic brain injury undergoing craniotomy or craniectomy. Neurosurgical focus. 2019 Nov 1;
[PubMed PMID: 31675713]
[38]
Bilotta F,Rosa G, 'Anesthesia' for awake neurosurgery. Current opinion in anaesthesiology. 2009 Oct;
[PubMed PMID: 19623055]
Level 3 (low-level) evidence
[39]
Fang S,Li Y,Wang Y,Zhang Z,Jiang T, Awake craniotomy for gliomas involving motor-related areas: classification and function recovery. Journal of neuro-oncology. 2020 Apr 29;
[PubMed PMID: 32350781]
[40]
O'Neill M,Henderson M,Duffy OM,Kernohan WG, The emerging contribution of speech and language therapists in awake craniotomy: a national survey of their roles, practices and perceptions. International journal of language
[PubMed PMID: 31778003]
Level 3 (low-level) evidence
[41]
Özlü O, Anaesthesiologist's Approach to Awake Craniotomy. Turkish journal of anaesthesiology and reanimation. 2018 Aug;
[PubMed PMID: 30140530]
[43]
Lu VM,Phan K,Rovin RA, Comparison of operative outcomes of eloquent glioma resection performed under awake versus general anesthesia: A systematic review and meta-analysis. Clinical neurology and neurosurgery. 2018 Jun;
[PubMed PMID: 29655013]
Level 1 (high-level) evidence
[44]
Chui J,Mariappan R,Mehta J,Manninen P,Venkatraghavan L, Comparison of propofol and volatile agents for maintenance of anesthesia during elective craniotomy procedures: systematic review and meta-analysis. Canadian journal of anaesthesia = Journal canadien d
[PubMed PMID: 24482247]
Level 1 (high-level) evidence
[45]
Guilfoyle MR,Helmy A,Duane D,Hutchinson PJA, Regional scalp block for postcraniotomy analgesia: a systematic review and meta-analysis. Anesthesia and analgesia. 2013 May;
[PubMed PMID: 23477962]
Level 1 (high-level) evidence
[46]
Mofatteh M,Mashayekhi MS,Arfaie S,Chen Y,Mirza AB,Fares J,Bandyopadhyay S,Henich E,Liao X,Bernstein M, Augmented and virtual reality usage in awake craniotomy: a systematic review. Neurosurgical review. 2022 Dec 19;
[PubMed PMID: 36529827]
Level 1 (high-level) evidence
[47]
Badenes R,Prisco L,Maruenda A,Taccone FS, Criteria for Intensive Care admission and monitoring after elective craniotomy. Current opinion in anaesthesiology. 2017 Oct;
[PubMed PMID: 28682828]
Level 3 (low-level) evidence
[48]
Bui JQ,Mendis RL,van Gelder JM,Sheridan MM,Wright KM,Jaeger M, Is postoperative intensive care unit admission a prerequisite for elective craniotomy? Journal of neurosurgery. 2011 Dec;
[PubMed PMID: 21888476]
[49]
Hanak BW,Walcott BP,Nahed BV,Muzikansky A,Mian MK,Kimberly WT,Curry WT, Postoperative intensive care unit requirements after elective craniotomy. World neurosurgery. 2014 Jan;
[PubMed PMID: 23182731]
[50]
Kulikov A,Tere V,Sergi PG,Bilotta F, Prevention and treatment of postoperative pain in pediatric patients undergone craniotomy: Systematic review of clinical evidence. Clinical neurology and neurosurgery. 2021 Apr 1;
[PubMed PMID: 33857811]
Level 1 (high-level) evidence
[51]
Collen JF,Jackson JL,Shorr AF,Moores LK, Prevention of venous thromboembolism in neurosurgery: a metaanalysis. Chest. 2008 Aug;
[PubMed PMID: 18641095]
[52]
Stumpo V,Staartjes VE,Quddusi A,Corniola MV,Tessitore E,Schröder ML,Anderer EG,Stienen MN,Serra C,Regli L, Enhanced Recovery After Surgery strategies for elective craniotomy: a systematic review. Journal of neurosurgery. 2021 May 7;
[PubMed PMID: 33962374]
Level 1 (high-level) evidence
[53]
Siegemund M,Steiner LA, Postoperative care of the neurosurgical patient. Current opinion in anaesthesiology. 2015 Oct;
[PubMed PMID: 26263123]
Level 3 (low-level) evidence
[54]
Cheng G,Hao S,Ye Z,Wang B,Huangpu B,Zhang P,Wang H,Hao Q, Potential risk analysis and experience summarization of unstable factors of cranial fixation devices in neurosurgical operations: three-case reports and systematic review. Chinese neurosurgical journal. 2021 Apr 28;
[PubMed PMID: 33910652]
Level 3 (low-level) evidence
[55]
Vaca EE,Purnell CA,Gosain AK,Alghoul MS, Postoperative temporal hollowing: Is there a surgical approach that prevents this complication? A systematic review and anatomic illustration. Journal of plastic, reconstructive
[PubMed PMID: 27894915]
Level 1 (high-level) evidence
[56]
Beniwal M,Shukla D, Management of Perforator Plunge in the Transverse Sinus. Pediatric neurosurgery. 2016;
[PubMed PMID: 27193189]
[57]
Lee CH,Koo HW,Han SR,Choi CY,Sohn MJ,Lee CH, Phenytoin versus levetiracetam as prophylaxis for postcraniotomy seizure in patients with no history of seizures: systematic review and meta-analysis. Journal of neurosurgery. 2019 Jun 1;
[PubMed PMID: 30004278]
Level 1 (high-level) evidence
[59]
Chughtai KA,Nemer OP,Kessler AT,Bhatt AA, Post-operative complications of craniotomy and craniectomy. Emergency radiology. 2019 Feb;
[PubMed PMID: 30255407]
[60]
Schipmann S,Akalin E,Doods J,Ewelt C,Stummer W,Suero Molina E, When the Infection Hits the Wound: Matched Case-Control Study in a Neurosurgical Patient Collective Including Systematic Literature Review and Risk Factors Analysis. World neurosurgery. 2016 Nov;
[PubMed PMID: 27506410]
Level 2 (mid-level) evidence
[61]
Fang C,Zhu T,Zhang P,Xia L,Sun C, Risk factors of neurosurgical site infection after craniotomy: A systematic review and meta-analysis. American journal of infection control. 2017 Nov 1;
[PubMed PMID: 28751035]
Level 1 (high-level) evidence
[62]
Alexandre V,Guyonaud C,Frasca D,Dahyot-Fizelier C, Major complications after scheduled craniotomy: A justification for systematic postoperative intensive care admission? European journal of anaesthesiology. 2020 Feb;
[PubMed PMID: 31913939]
Level 1 (high-level) evidence
[63]
Alotaibi AF,Hulou MM,Vestal M,Alkholifi F,Asgarzadeh M,Cote DJ,Bi WL,Dunn IF,Mekary RA,Smith TR, The Efficacy of Antibacterial Prophylaxis Against the Development of Meningitis After Craniotomy: A Meta-Analysis. World neurosurgery. 2016 Jun;
[PubMed PMID: 26921699]
Level 1 (high-level) evidence
[64]
Reponen E,Tuominen H,Korja M, Evidence for the use of preoperative risk assessment scores in elective cranial neurosurgery: a systematic review of the literature. Anesthesia and analgesia. 2014 Aug;
[PubMed PMID: 25046789]
Level 1 (high-level) evidence
[65]
Bilotta F,Guerra C,Rosa G, Update on anesthesia for craniotomy. Current opinion in anaesthesiology. 2013 Oct;
[PubMed PMID: 23995058]
Level 3 (low-level) evidence