Continuing Education Activity
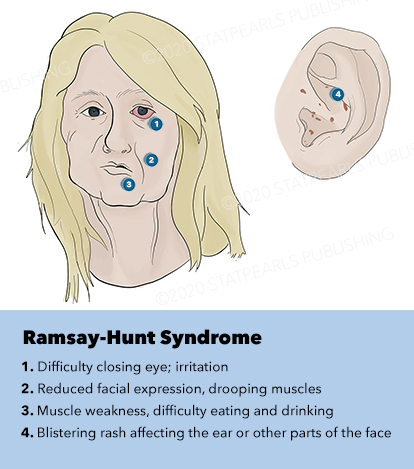
Ramsay Hunt syndrome, also known as herpes zoster oticus, is a late complication of varicella-zoster virus infection that results in inflammation of the geniculate ganglion of cranial nerve VII. Ramsay Hunt is a clinical diagnosis and classically is described as a triad of ipsilateral facial paralysis, otalgia, and vesicles near the ear and auditory canal. Diagnosis is often missed or delayed, which can lead to an increased incidence of long-term complications. The condition is self-limiting, but treatment is targeted at decreasing the total duration of the illness as well as providing analgesia and preventing the complications that can occur. This activity reviews the role of the interprofessional team in the diagnosis and treatment of Ramsay Hunt syndrome.
Objectives:
- Identify the clinical signs and symptoms of Ramsay Hunt syndrome.
- Review the evaluation of patients with suspected Ramsay Hunt syndrome.
- Outline the management options available for Ramsay Hunt syndrome.
- Discuss interprofessional team strategies for improving care coordination and communication to improve patient outcomes for Ramsay Hunt syndrome.
Introduction
Ramsay Hunt syndrome, also known as herpes zoster oticus or geniculate ganglion herpes zoster, is a late complication of varicella-zoster virus (VZV) infection, resulting in inflammation of the geniculate ganglion of cranial nerve VII.[1] The syndrome is named after James Ramsay Hunt (1872-1937), an American neurologist and Army officer in World War I who described three different syndromes, the most famous of which is the second, which is discussed herein as "Ramsay Hunt syndrome."[2] Early stages of VZV infection cause fever and diffuse vesicular rash, a condition that is commonly referred to as chickenpox. After the initial infection, the virus will often remain dormant in the body. Subsequent reactivation of the virus causes a "zoster" or "herpes zoster" phenomenon. This syndrome consists of pain and a vesicular rash along the involved nerve's distribution, typically corresponding to a single dermatome. The distribution and associated symptoms depend on the nerve involved. Less than 1% of zoster cases involve the facial nerve and result in Ramsay Hunt syndrome.[3]
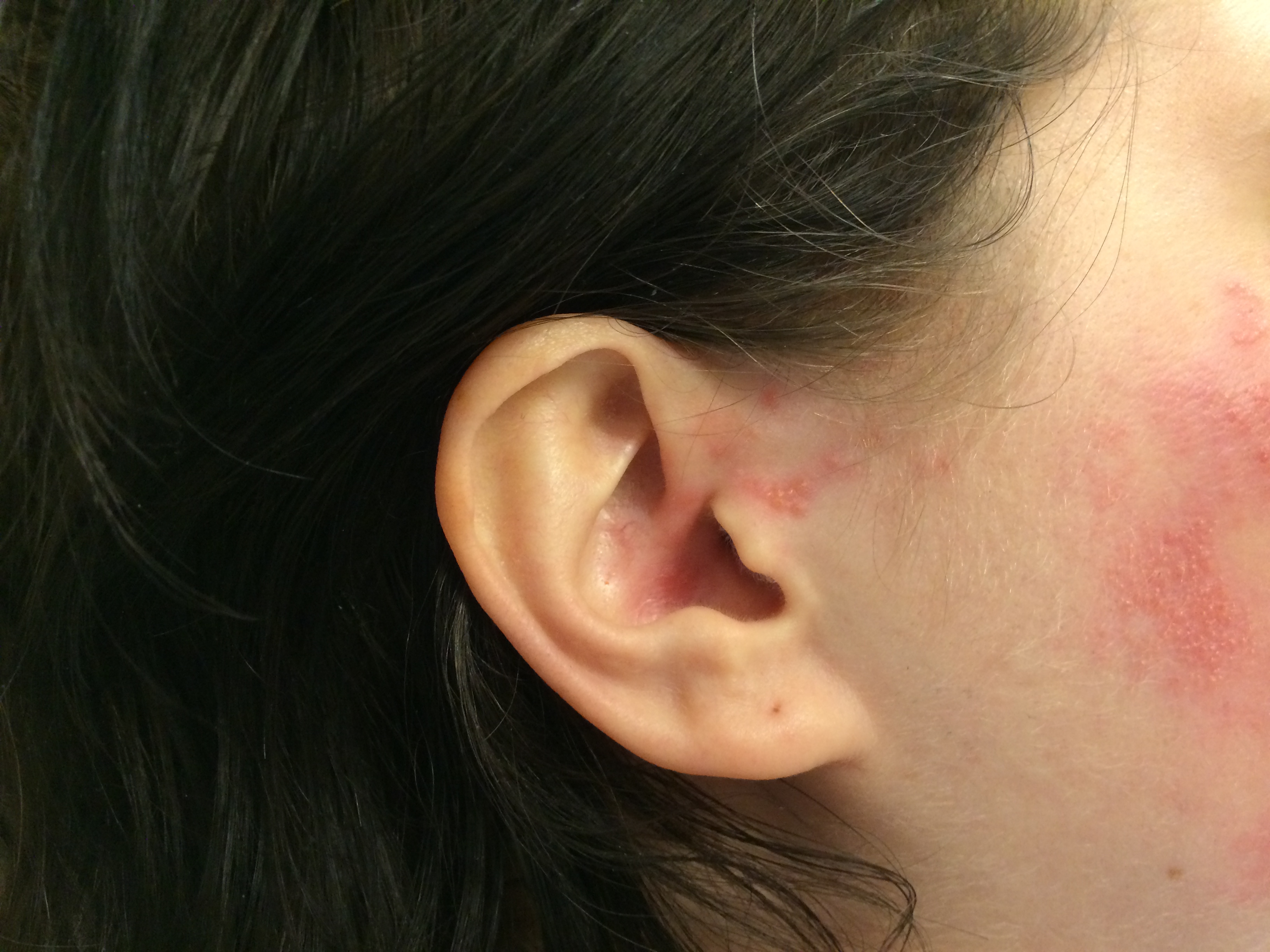
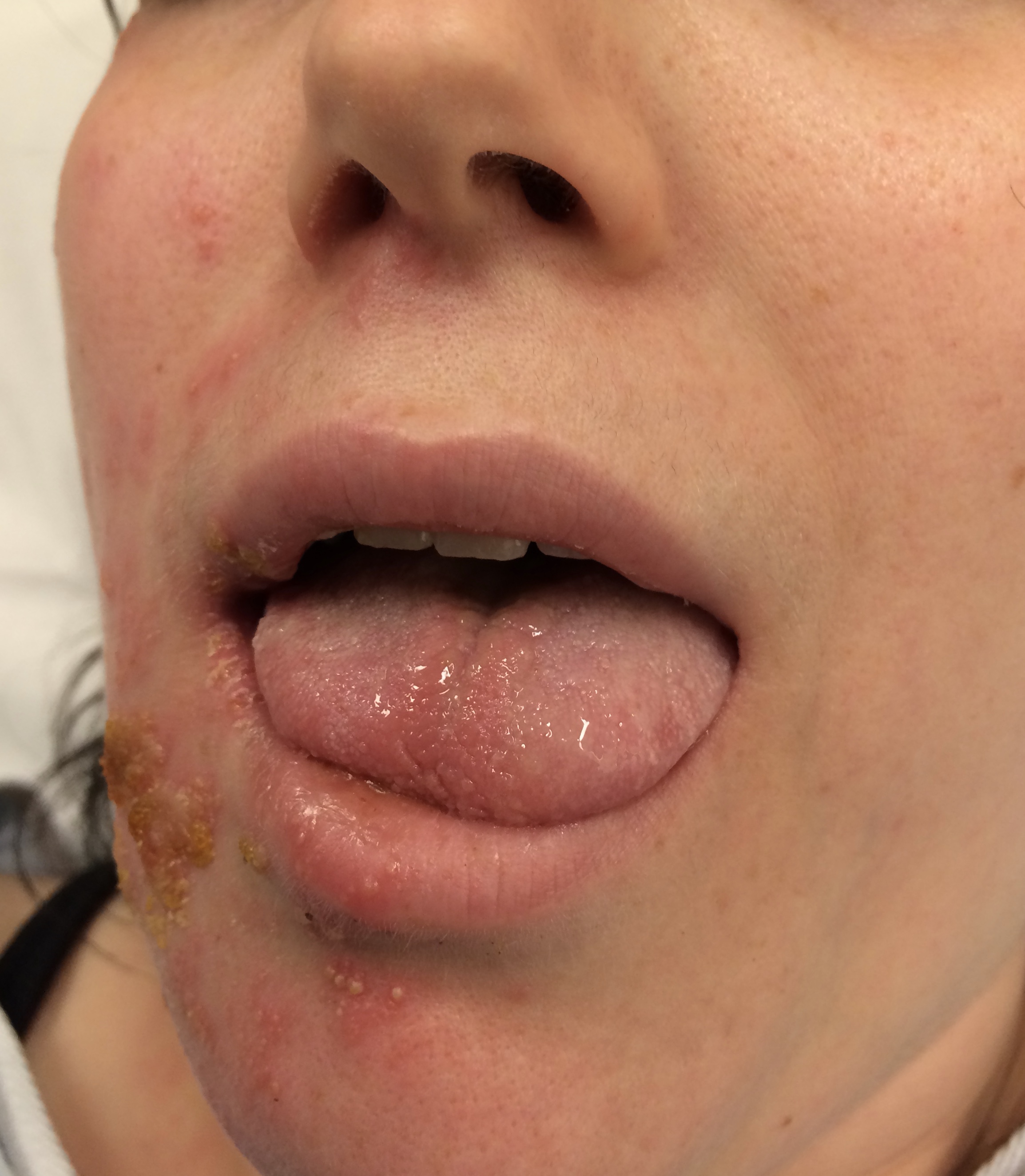
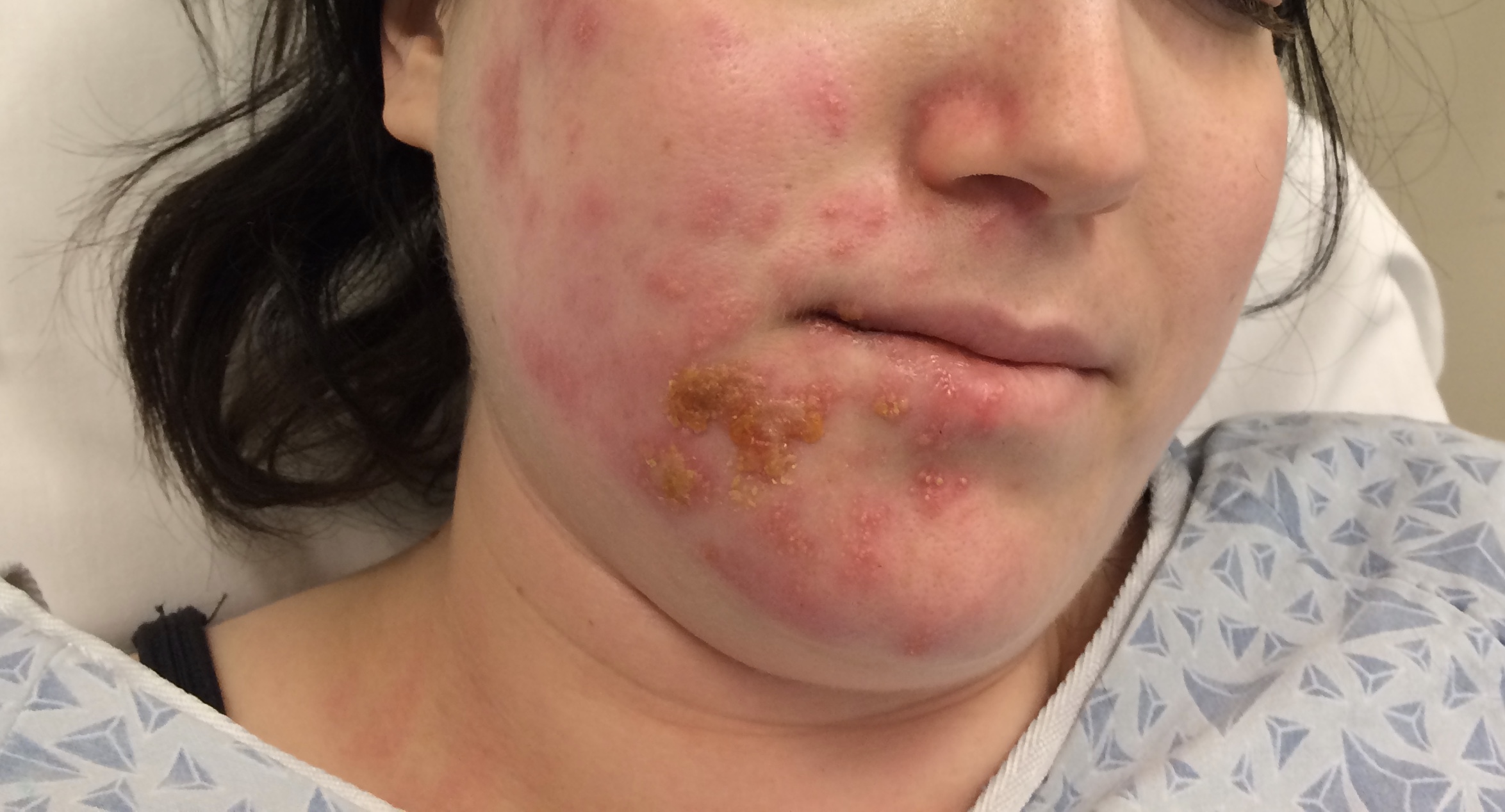
Although the classic triad of Ramsay Hunt syndrome is ipsilateral facial paralysis, otalgia, and a vesicular rash, there is significant variability in clinical presentation, with some patients demonstrating facial paralysis before the rash or, sometimes, no rash at all.[4][5][4] In the latter, the patient's chief complaints are severe ear pain and facial weakness; this variant is known as zoster sine herpete and can be very difficult to clinically distinguish from Bell's palsy. Zoster sine herpete has been reported to comprise up to 30% of Ramsay Hunt cases.[6] If a rash is present, it may be frankly vesicular or maculopapular, and can involve the affected side of the face, scalp, palate, and tongue. Additional symptoms that may be reported include a change in taste sensation, dry eye, tearing, hyperacusis, nasal obstruction, and dysarthria. Hearing loss, tinnitus, and vertigo can be seen with involvement of the vestibulocochlear nerve, and hoarseness or aspiration may indicate involvement of the vagus nerve.
Etiology
The causative agent in Ramsay Hunt syndrome is the varicella-zoster virus, a member of the human herpesvirus family. More specifically, it is part of the alphaherpesvirinae subfamily, along with herpes simplex viruses 1 and 2 (HHV-1 and HHV-2). VZV is a double-stranded DNA virus, more technically known as human alphaherpesvirus 3 (HHV-3).[7] Once the clinical VZV infection, chickenpox, has cleared, the virus remains latent in cranial nerves or dorsal root ganglia and may subsequently reactivate in times of physiological stress or immunocompromise, leading to herpes zoster, known as "shingles" anywhere on the body or "Ramsay Hunt syndrome" when facial paralysis is involved.[1] While the incidence of chickenpox and shingles have decreased dramatically since VZV vaccination became widely available in 1995, shingles and Ramsay Hunt syndrome have nevertheless been reported in patients who have never contracted chickenpox but who have been vaccinated with live attenuated VZV.[8][9][10]
Epidemiology
Ramsay Hunt syndrome affects both immunocompetent and immunocompromised patients and has an incidence of about 5 per 100,000 people per year; in contrast, the incidence of Bell palsy is much higher, at about 15-30 per 100,000 people per year.[11][12] Ramsay Hunt syndrome accounts for roughly 7% of acute facial paralysis cases, with zoster sine herpete comprising up to 30% of those.[6] Immunocompromised patients are likely to have a more severe disease process and less complete recovery. Ramsay Hunt syndrome can present in anyone, and there are cases reported in patients ranging from 3 months of age to 82 years, although patients in their 7th and 8th decades are most susceptible.[13] Factors that increase the risk of herpes zoster will similarly increase the incidence of Ramsay Hunt syndrome, including stress, chemotherapy, immunocompromise, infection, malnutrition, among others.
Pathophysiology
Initial infection with the varicella-zoster virus causes a disseminated vesicular rash with fever, known as chickenpox. During the acute phase of this infection, the virus is spread by respiratory droplets. After the viremia and exanthem have resolved, the virus can remain dormant in cranial nerves and dorsal root ganglia. During times of physiological stress or immunocompromise, reactivation of the virus within the distribution of the nerve in which it has been dormant can occur. In Ramsay Hunt syndrome, the virus lays dormant within the geniculate ganglion and primarily reactivates along the facial nerve, although other cranial nerves may be involved.
According to Coulson et al., the initial presenting symptom is typically pain in the ipsilateral ear (55% of patients), with facial paralysis and vesicles appearing within 2 to 3 days. In 23% of patients, facial paralysis is the presenting symptom, and in only 2%, vesicles appear first. While 86% of their patients reported that the rash occurred only on the auricle, 7% only had vesicles in the oral cavity, and 8% had them in both locations.[14] Rashes have also been reported on the scalp and the cheek.
The proximity of the facial nerve to the vestibulocochlear nerve can result in hearing loss, tinnitus, and vertigo. Sensorineural hearing loss was present in 43% of patients in Coulson's series, imbalance or vertigo in 51%, and tinnitus in 20%.[14] Vagal nerve involvement is also probably more common than it appears to be. Unless the patient is symptomatic with hoarseness or aspiration, vocal cord paralysis is not usually noted because it requires mirror or fiberoptic laryngoscopy to discover.[15][16] Although less frequent, other cranial nerves that can be involved include the trigeminal, glossopharyngeal, and hypoglossal nerves. Cranial polyneuropathy is more likely to present in immunocompromised patients, such as those with diabetes mellitus or human immunodeficiency virus infection.[17][18][19][15]
The facial paralysis resulting from Ramsay Hunt syndrome has a worse prognosis than that seen in Bell's palsy, with only 70% regaining normal or near-normal facial function compared with over 90% in Bell's palsy.[20][21] After Bell's palsy, the rate of synkinesis development is roughly 16%, but it is closer to 40% after Ramsay Hunt syndrome.[22][23] The neuritis associated with Ramsay Hunt syndrome appears to be more severe than that of Bell's palsy, given that more than twice as many patients present with complete hemifacial paralysis with Ramsay Hunt syndrome as in Bell's palsy.[14][22]
When patients fail to recover premorbid function, synkinesis is a common sequela, in which aberrant reinnervation connects axons with neuromuscular junctions differently from the ones to which they were connected prior to the inflammation. Severe neuritis and resultant swelling of the facial nerve against the bony Fallopian canal that encases it within the temporal bone causes direct, physical neuronal injury.[24] Consequently, Wallerian degeneration of facial nerve axons occurs along with varying degrees of damage to the internal architecture of the nerve. When the endoneurium, perineurium, or epineurium are disrupted (as in Sunderland class III-V injuries), the axons no longer have a physical structure in place to guide reinnervation and may ultimately connect with the incorrect muscle or end-organ. In synkinesis, newly regenerated axons will terminate at multiple neuromuscular junctions, which has the effect of further increasing dyscoordination as well as raising the resting tone of the muscles that now have more associated axons than they did before the injury. Patients with synkinesis often complain of eye closure with mouth movements and vice versa, as well as facial tension and pain. Because the facial nerve supplies parasympathetic fibers to the lacrimal glands, patients may also develop gustatory lacrimation, or Bogorad syndrome, due to aberrant reinnervation; this causes tearing with eating. All of these issues, as well as the physical difficulties associated with acute facial paralysis, can have a significant negative impact on a patient's quality of life.[25]
Histopathology
Microscopic evaluation with the Tzanck smear technique can be performed on the fluid obtained from the vesicles. This should reveal multinucleated giant cells using a Giemsa stain, methylene blue, or Wright's stain. The sensitivity of the test is low, but it has a high specificity when pre-test clinical suspicion is high. Distinguishing between herpes simplex, varicella-zoster, and cytomegalovirus infections can be challenging because they are all herpesviruses; the Tzanck smear is also used to identify pemphigus vulgaris, leprosy, and leishmaniasis.[26]
History and Physical
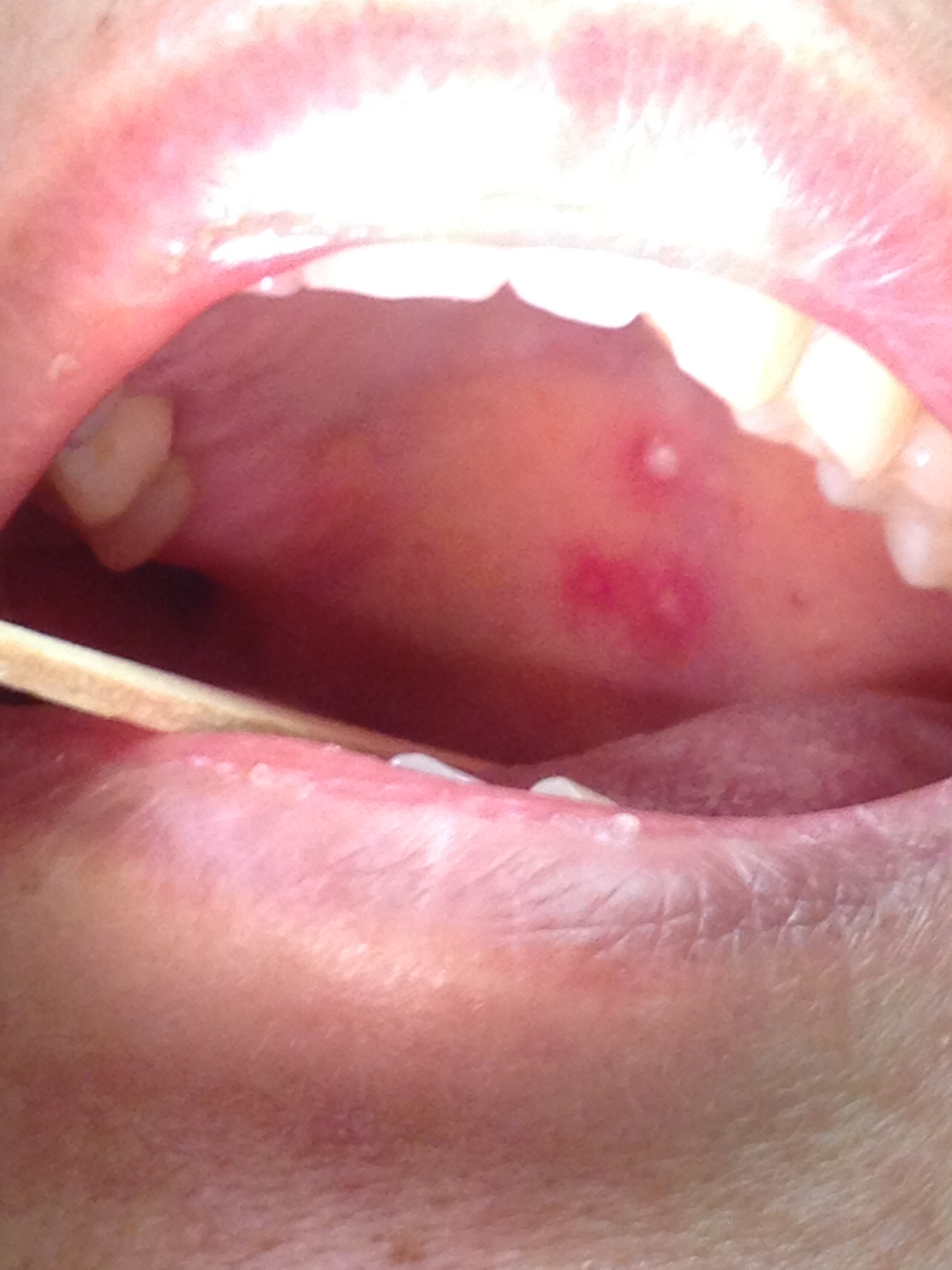
Ramsay Hunt patients typically present with the classic triad of ipsilateral facial paralysis, otalgia, and painful vesicles on the auricle; however, patients who present early in the course of the disease may only have pain without facial paralysis or a rash. Notably, the characteristic rash correlates to the areas innervated by the facial nerve; on the auricle, this includes the conchal bowl, anti-helix, and postauricular sulcus, but the rash can involve the auditory canal, scalp, cheek, tongue, or palate.[4] Often, there is a prodrome with pain, non-specific headache, fever, and fatigue for 1 to 3 days, followed by the onset of facial paralysis and exanthem.[14] Facial palsy typically takes 1 to 3 days to reach its nadir, with more rapid development often resulting in more severe paralysis. The rash generally begins as erythematous papules and then progresses to frank vesicles, which subsequently rupture and crust over within 1 to 7 days. The timing of the rash relative to facial paralysis is variable, and it may appear after the palsy.[13] Lesions can persist for 2 to 3 weeks, leaving erythematous macular scars.[13]
Although the presence of rash is variable, the majority of Ramsay Hunt patients report pre-herpetic, or pre-eruptive, otalgia. This is most frequently described as severe, of a sharp or stabbing quality, may awaken the patient from sleep, and persists for multiple days.[27] Patients will often complain of a myriad of other symptoms during the acute phase of the syndrome: hyperacusis, dysgeusia, nasal obstruction, epiphora, xerophthalmia, drooling, dysarthria, smile asymmetry, vertigo, tinnitus, hearing loss, hoarseness, dysphagia, and facial numbness. This list reflects the broad range of functions controlled by the cranial nerves potentially affected in Ramsay Hunt syndrome: V, VII, VIII, IX, X, XII.[4] When severe pain precedes facial palsy in the absence of a vesicular rash, patients are diagnosed with zoster sine herpete, which accounts for up to 30% of Ramsay Hunt syndrome presentations, and can be quite challenging to differentiate from Bell's palsy, which also often presents with otalgia, though typically not as severe.[28] Patients should also be asked about emotional status, as depression commonly accompanies facial palsy in some demographic groups, particularly young women.[25]
Generally, the most apparent feature on physical examination will be unilateral hemifacial palsy, which will be complete in close to 50% of patients.[14] Facial analysis may reveal the absence of transverse forehead rhytides, brow ptosis, lagophthalmos, ectropion, effacement of the nasolabial fold, the collapse of the nasal valve, malposition of the oral commissure inferiorly, and absence of platysmal banding on the affected side. The philtrum and nasal base will also likely shift away from the paralyzed side. Examination of a facial paralysis patient should be performed in three distinct passes. The first should look for asymmetry at rest, evaluating the upper third of the face, followed by the middle, and then the lower face and neck. The second should assess each of the major extratemporal branches of the facial nerve in sequence: frontal by brow elevation, zygomatic by gentle and forceful eye closure, buccal by smiling, marginal mandibular by depression of the lower lip, and cervical by platysmal contraction. Evaluating each of these movements also makes grading and documentation using the House-Brackmann scale straightforward. In the House-Brackmann system, a grade I represents normal facial function, grade II slight asymmetry with movement but complete eye closure with gentle effort, grade III more asymmetry with movement but complete eye closure with maximal effort only, grade IV still greater asymmetry with movement and inability to close the eye completely, grade V gross resting asymmetry and inability to close the eye, and grade VI demonstrates no movement at all.[29] Numerous other clinical facial nerve grading systems are available, but the House-Brackmann scale remains the most widely employed due to its ease of use.[30][31][32]
The third pass of the facial examination should repeat the same movements as the second pass, but the examiner should look for involuntary movements in other areas of the affected hemiface. These synkinetic movements, commonly seen as twitching of the mouth during eye closure or winking during a smile, indicate aberrant regeneration and do not typically appear until 4-6 months after the onset of the paralysis; they are more common in patients with more severe initial paralysis.[22] For documentation of longitudinal follow-up, it is helpful to take photographs and videos of patients performing the standard maneuvers of the facial nerve exam.[33]
Patients with lagophthalmos should have their Bell's phenomenon evaluated, as this is an important corneal protection mechanism. The Bell's phenomenon is the upward rotation of the globe when the eyelids close; it is often lost as patients age. Patients who complain of eye pain or a foreign body sensation should undergo a slit-lamp examination to assess for corneal abrasions or exposure keratopathy, which are very common in cases of facial paralysis. Assessment of corneal sensation is also important because even though corneal anesthesia is rare in Ramsay Hunt syndrome, patients will be predisposed to ocular injury if the eye does not close properly and foreign bodies cannot be felt. Patients with hearing or balance complaints should undergo audiometry and potentially vestibular testing, and patients who note hoarseness or dysphagia should undergo fiberoptic laryngoscopy to evaluate the vocal cords. All patients should receive a thorough cranial nerve examination and inspection of the oral cavity, scalp, external ears, and auditory canals to look for a vesicular eruption. The nose and eyes are not typically involved with vesicles in Ramsay Hunt syndrome, but this can be seen in other forms of zoster. Vesicles near the tip of the nose, Hutchinson's sign, can be associated with ocular lesions.
Evaluation
Ramsay Hunt syndrome is a clinical diagnosis, as laboratory testing to confirm the presence of VZV is often impractical and lacks sensitivity.[13] VZV can be cultured from vesicular fluid, blood, saliva, or tears. Polymerase chain reaction (PCR) assays have been reported to have a sensitivity for VZV of approximately 58%. Enzyme-linked immunoassays (ELISA) have sensitivities as high as 82-99% but have limited utility in the acute setting, as anti-VZV antibodies take weeks to develop and may be confounded by the patient's vaccination status.[6] Magnetic resonance imaging (MRI) frequently demonstrates inflammation near the geniculate ganglion of the affected facial nerve, but computed tomography (CT) generally does not contribute to the diagnosis, nor is the MRI strictly necessary. Audiometry, vestibular testing, and flexible fiberoptic laryngoscopy may help determine the extent of cranial nerve involvement. When the cause of acute facial paralysis is unclear clinically, imaging and serological studies may be helpful, but most cases of Ramsay Hunt syndrome are diagnosed with a thorough history and physical exam.[28]
When available, electrodiagnostic testing such as electroneuronography (ENoG) and electromyography (EMG) may provide useful prognostic information by quantifying the extent of nerve damage more precisely than is possible with a physical examination alone, predominantly in the case of House-Brackmann grade VI paralysis.[34][35]
Treatment / Management
Herpes zoster is generally self-limiting in nature. Therefore, the main goals of treatment are to decrease the incidence of late complications, including spastic facial paralysis and postherpetic neuralgia. Multiple studies have shown a significant decrease in long-term complications with the use of oral antivirals and steroids.[20][14] It is unclear, however, whether these medications decrease the length or severity of acute symptoms. Acyclovir, valacyclovir, and famciclovir have all been studied and found effective. Acyclovir, 500 mg five times a day, is usually the most affordable option. Valacyclovir, 1000 mg three times a day, is easier for most patients to take and appears to be more effective, at least in Bell's palsy.[36] Another option is famciclovir, 500 mg three times a day, which also appears to be more effective than acyclovir.[37] Antiviral treatment is usually administered for 7 to 10 days; however, some studies have reported prolonged or delayed degeneration of facial nerve axons up to 21 days after paralysis onset, and therefore recommend continuing antiviral therapy for 21 days.[1][14]
High-dose corticosteroids, either oral or intravenous, should be co-administered with antiviral treatment. There is no overall consensus regarding the total duration of steroid treatment, which can vary from 4 to 37 days, but steroids should be prescribed at a high dose.[20] This is typically prednisone 1 mg/kg/day up to a maximum dose of 60 mg, or the equivalent of such, followed by a taper to prevent acute adrenal insufficiency. In a cohort of Ramsay Hunt patients with complete facial paralysis at initial presentation, Furukawa et al. demonstrated a potential benefit of even higher doses of corticosteroid, up to 200 mg with a subsequent 10-day taper, in combination with antiviral therapy.[38] Another modality for corticosteroid treatment is via intratympanic injection. Cadaveric studies have demonstrated dehiscence of the intratympanic segment of the facial nerve in 50-80% of patients.[39] This dehiscence allows for topical administration of a corticosteroid, sparing the patient from the adverse side effects associated with systemic steroids. In one of the few prospective studies on Ramsay Hunt patients, Inagaki et al. showed that concurrent treatment with systemic and daily intratympanic steroid injections for 10 days increased the rate of recovery, with 11 out of 12 patients who initially demonstrated House-Brackmann IV-VI facial paralysis exhibiting full recovery to House-Brackmann I when evaluated 12 months after symptom onset.[40] In a recent meta-analysis evaluating the efficacy of concurrent treatment in Ramsay Hunt syndrome and Bell's palsy patients, those who received intratympanic steroid injections, in addition to systemic steroids, were found to have a relative reduction in the risk of non-recovery by 64%.[39] Prospective studies are still necessary to confirm the efficacy of intratympanic steroid injections in acute facial paralysis but may be a reasonable adjunct to treatment in Ramsay Hunt patients with moderate to severe facial paralysis.
Notably, the clinician should have an appropriate and comprehensive patient-specific discussion before prescribing high-dose corticosteroids. Common side effects include mood changes such as depression or irritability, insomnia, gastritis, gastroesophageal reflux, increased blood pressure, and hyperglycemia.[9] Although diabetes is not an absolute contraindication to steroid prescription, these patients may have difficulty tolerating steroids at high doses and should adjust their hypoglycemic medications appropriately. In some cases, temporary use of insulin may be necessary rather than forgoing steroid treatment because the combination of glucocorticoids and antiviral drugs has been shown to be more effective than antivirals alone.[20] Prophylactic addition of a proton pump inhibitor or misoprostol may be considered for further gastrointestinal protection while taking high-dose corticosteroids. Treatment should be started as soon as possible, but initiating therapy as late as one week after symptom onset is still helpful for patients who present in a delayed fashion.
Symptomatic management is also critical, particularly for two aspects of Ramsay Hunt syndrome: pain and corneal exposure. Analgesia is often needed with zoster; a multimodal approach with acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), and long-acting opioids can all be used. If steroids are prescribed, concomitant NSAID use should generally be avoided to mitigate the risk of enteropathy, ulcer formation, and subsequent gastrointestinal bleeding. Tricyclic antidepressants and gabapentin are useful for the treatment of neuropathic pain and postherpetic neuralgia. Meclizine and benzodiazepines can be effective for managing acute vertigo as well. Artificial tears throughout the day and ocular lubricant ointment at night are helpful for the prevention of exposure keratopathy, and patients with frank lagophthalmos should be instructed in how to stretch the eyelid and how to tape the eye closed at night in such a way as to avoid scratching the cornea in the process (see videos). Patients with multiple comorbidities, such as diabetes, old age, hypertension, and immunocompromise, may take longer to recover than most, and they may benefit from the placement of an upper eyelid weight to aid eye closure and prevent exposure keratopathy during the recovery period.[41] Eyelid weight placement is particularly indicated in patients with corneal hypesthesia.[28]
Further surgical intervention in Ramsay Hunt patients in the acute setting is controversial. Generally, surgical decompression is considered to be beneficial in Bell's palsy patients if electrodiagnostic testing shows >90% degeneration of the facial nerve without the presence of voluntary motor potentials, the affected side is completely paralyzed, and if surgery is performed within 14 days from paralysis onset.[42] Whether or not these same parameters can be applied to prognosticate patients with Ramsay Hunt syndrome is unclear. Some surgeons may choose to offer surgical decompression to patients within the 14-day time period, or even up to 50 days after onset.[42] Although the role of surgical intervention in improving recovery in Ramsay Hunt syndrome is unclear, it should be emphasized that decompression should only be offered to select patients with a poor prognosis at baseline. Due to the relatively rare number of Ramsay Hunt cases, and even rarer number of Ramsay Hunt patients who fail to show some degree of recovery during or after medical treatment, it has been difficult to draw conclusions regarding the optimal candidacy and timing of surgery. Surgical decompression of the facial nerve has been achieved via either a transmastoid or middle cranial fossa approach.
In the long term, the management of synkinesis can be accomplished with both conservative and surgical approaches.[43] Conservative approaches include massage and physical therapy as well as chemodenervation with botulinum toxin. Surgical management of synkinesis may involve selective neurectomy and/or myomectomy, or even nerve or functional free muscle transfer to improve smile symmetry.[44][45][46][47][48]
Differential Diagnosis
Not many clinical entities produce both a facial rash and paralysis, but other common causes of localized facial rashes include herpes simplex virus outbreaks, Staphylococcal impetigo, and contact dermatitis that may arise from topical agents like neomycin or exposure to an irritant such as poison ivy. Acute facial paralysis is most commonly caused by Bell's palsy, which presents similarly to zoster sine herpete, but other non-traumatic etiologies include Lyme disease, benign skull base tumors, extratemporal malignancies, autoimmune conditions, otologic disease, other viral infections, neurosyphilis, and stroke.[13][28] While cortical strokes classically spare the forehead, brainstem strokes cause paralysis of the entire hemiface; regardless, an evolving stroke should present with vital sign instability and neurological symptoms other than facial paralysis. Multiple cranial neuropathies can occur in the setting of central nervous system pathology as well as viral infections, including SARS-CoV-2.[49][50]
Prognosis
All patients recover from Ramsay Hunt syndrome; the question is, "to what degree?" Overall, the most consistent prognostic indicator in Ramsay Hunt syndrome is the presenting severity of facial paralysis. Patients who present with House-Brackmann grade III paralysis tend to recover to normal function; patients with House-Brackmann grade IV or V paralysis are more likely to recover to grade II function, and patients with House-Brackmann grade VI function at presentation are more likely to recover to grade III function.[14] Patients who do not recover their premorbid function will almost certainly develop some degree of synkinesis. Clinically significant flaccid paralysis is extremely rare in the long term. Most patients complete their recovery within one year. Relatively young, healthy patients with incomplete paralysis will often recover to full or near-full function within several weeks to a few months. Overall, roughly 70% of Ramsay Hunt patients will recover to House-Brackmann grade I or II function.[14][20][14] In general, the prognosis for Ramsay Hunt syndrome is worse than that for Bell's palsy, which has a lower rate of synkinesis development and failure to return to premorbid function.[51] In addition to synkinesis, an unfortunate sequela of Ramsay Hunt syndrome is postherpetic neuralgia, pain present for longer than three months after onset, which is more likely to develop in patients older than 50 years and patients who experience facial numbness in the acute period.[52]
Other factors that have been associated with non-recovery include age over 50 years old, a greater degree of axonal damage on electrodiagnostic testing, involvement of multiple cranial neuropathies, the presence of oropharyngeal lesions, and diabetes.[4][20][23][53][14][53] The presence of the characteristic rash before the onset of facial palsy, which occurs in about 25% of Ramsay Hunt patients, seems to portend a better prognosis.[38] There has been growing interest in the role of peripheral biomarkers, particularly the calculated neutrophil-to-lymphocyte ratio (NLR), as an initial prognostic indicator in acute facial palsy.[54][55][56][57][58] This is particularly relevant as inflammation and subsequent swelling of the facial nerve likely causes more physical trauma than VZV reactivation itself, but cannot be quantified until Wallerian degeneration is complete at a minimum of 72 hours after onset of paralysis. In a recent meta-analysis, both Bell's palsy and Ramsay Hunt patients who exhibited a higher pre-treatment NLR were less likely to achieve full or near-full recovery.[58] The role that NLR may have in providing patient-specific prognostication at initial presentation is still largely speculative and larger, well-designed, prospective studies are necessary to clarify its utility.
Complications
Other than the presenting symptoms of pain, rash, facial paralysis, dysgeusia, hearing loss, tinnitus, vertigo, hoarseness, dysarthria, and others mentioned above, short-term complications of Ramsay Hunt syndrome include corneal abrasion and exposure keratopathy, depression and social anxiety, and transmission of chickenpox to unvaccinated or immunocompromised close contacts. While long-term flaccid paralysis is unlikely, the development of synkinesis is very common. Other long-term complications include postherpetic neuralgia, scarring from the vesicles, and persistent depression and/or social anxiety due to loss of facial function.
Consultations
While Ramsay Hunt syndrome is ideally managed pharmacologically by a primary care provider, specialist consultation with a physician with acute facial paralysis experience may be valuable. An otolaryngologist or facial plastic surgeon may be more familiar with the treatment of Ramsay Hunt syndrome than a general practitioner and likely has easier access to audiometry and flexible fiberoptic laryngoscopy. A neurologist is also helpful in evaluating cranial neuropathies, assisting with electrodiagnostic testing for patients with complete hemifacial paralysis, and may help treat chronic neuralgia. An ophthalmologist can evaluate the health of the cornea with slit lamp examination and fluorescein dye, and both the ophthalmologist and the otolaryngologist should be capable of placing an eyelid weight, if necessary. An internist or endocrinologist may be required to manage blood glucose levels or hypertension during prolonged high-dose steroid administration. Lastly, some patients will require consultation with a behavioral health specialist to assist with managing mood symptoms and anxiety that stem from facial dysfunction.
Deterrence and Patient Education
When discussing Ramsay Hunt syndrome with patients, it is essential to emphasize that everyone gets better, but not everyone gets all the way better. Explaining that "not all the way better" may involve synkinesis and/or postherpetic neuralgia is critical. Perhaps even more crucial is impressing upon patients the importance of corneal protection during the period of flaccid paralysis because the temporary nature of the facial palsy does not preclude the possibility of sustaining a permanent ocular injury during that interval. Accordingly, the application of artificial tears throughout the day and ocular lubricant ointment at night, and the use of eyelid stretching and taping can mean the difference between a satisfactory long-term outcome and an unsatisfactory one. It is also important for patients with active vesicles to avoid contact with unvaccinated and immunocompromised individuals, as they can spread the varicella-zoster virus from their lesions. Lastly, administering the shingles vaccine likely prevents Ramsay Hunt syndrome as well, but the vaccine is not 100% effective. It is also important to remember that while most patients will not develop chickenpox or zoster more than once, it has been reported, particularly in immunocompromised individuals.[59]
Enhancing Healthcare Team Outcomes
Ramsay Hunt syndrome affects patients in a myriad of ways, with pain, paralysis, cochleovestibular symptoms, and behavioral health concerns all occurring commonly in the acute period. While most patients do recover the majority of their premorbid function when managed appropriately, long-term pain, facial dysfunction, scarring, and behavioral health concerns may all persist. For this reason, optimal patient outcomes occur when healthcare teams include members with expertise across a broad range of specialties. In the short term, primary care, otolaryngology, neurology, ophthalmology, and psychology/psychiatry are often required. In the long-term, facial plastic surgery or otolaryngology, pain management, ophthalmology, speech or physical therapy, and psychology/psychiatry may be needed. Patients who develop synkinesis may require regular visits over the course of many years with a physician or nurse who can administer botulinum toxin injections; it is critical to surround these patients with an experienced interprofessional team early on in the treatment process in order to provide the care and support they need to maximize their quality of life outcomes.[Level 1]