Introduction
Smooth muscle is present throughout the body, where it serves a variety of functions. It is in the stomach and intestines, where it helps with digestion and nutrient collection. It exists throughout the urinary system, where it functions to help rid the body of toxins and works in electrolyte balance. It is present throughout arteries and veins, where it plays a vital role in the regulation of blood pressure and tissue oxygenation. Without these vital functions, the body would not be able to maintain even its most basic functions.
Smooth muscle differs from skeletal muscle in a variety of ways, perhaps the most important being its ability to be contracted and controlled involuntarily. The nervous system can use smooth muscle to tightly regulate many of the body's subsystems for life with no thought from the user. A person does not need to think about their blood pressure for it to adapt to increasing oxygen demands from exercise. The nervous system instead uses hormones, neurotransmitters, and other receptors to control smooth muscle spontaneously.
Smooth muscle also plays an essential role in the disease process throughout the body. The use of bronchodilators to relax airway smooth muscle is an important and life-saving treatment in asthmatics.[1] Likewise, medications like metoclopramide can stimulate and promote gastric emptying by increasing smooth muscle signaling. Perhaps one of the most well-known uses of medical therapy and smooth muscle is the use of nitrates in the treatment of ischemic heart disease.[2] Research showed that nitrates, in combination with ace inhibitors, can improve patient mortality.[3] The uniquely significant impact that smooth muscle has throughout the body makes it an important topic for medical professionals to understand as many treatments at their core rely on modifying the signaling pathways that affect smooth muscle.
Cellular Level
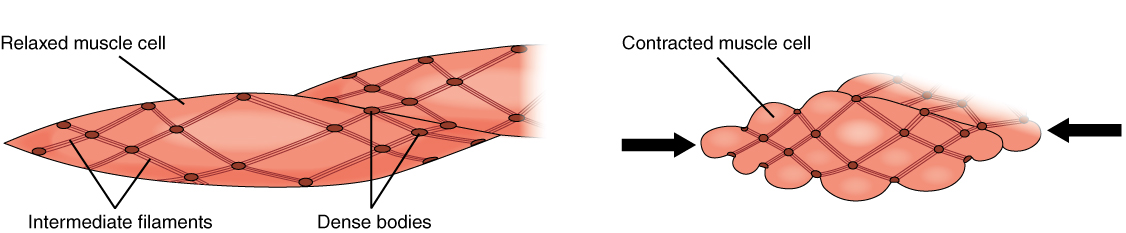
At a cellular level, smooth muscle functions as an involuntary non-striated muscle. Smooth muscle contains thick and thin filaments that do not arrange into sarcomeres, resulting in a non-striated pattern. On microscopic examination, it appears homogenous. Smooth muscle cytoplasm contains large amounts of actin and myosin. Actin and myosin act as the main proteins involved in muscle contraction. Actin filaments attach to dense bodies spread throughout the cell. Dense bodies can be observed under an electron microscope and appear dark. Another important structure is the calcium-containing sarcoplasmic reticulum, which aids in sustaining contraction. The shape of smooth muscle is fusiform, which is round in the center and tapering at each end. Smooth muscle can tense and relax but has greater elastic properties than striated muscle. This quality is important in organ systems like the urinary bladder, where the preservation of contractile tone is a necessity.
Development
Smooth muscle derives from both mesoderm and neural crest cells; this is because smooth muscle contributes to many different tissues throughout the body. One unique feature of neural crest cells is that their migration occurs during embryological development. For this reason, numerous tissues throughout the body originate from neural crest cells. Neural crest cells play a vital role in the development of smooth muscle throughout the body, specifically in the regulation of blood vessels.
Vascular smooth muscle cells arise from multiple origins; this becomes medically significant because they may contribute to the site-specific localization of vascular diseases. For example, atherosclerosis and aortic aneurysms often present at specific vascular locations. In the past, this appeared to be related to hemodynamics and underlying vessel structure. However, there is increasing evidence that smooth muscle cell embryonic lineage may play a role in determining the location and presentation of diseases.[4] Smooth muscle cell development is also an important factor in the development of the endothelial network. Vascular smooth muscle cells, sometimes referred to as mural cells, are essential for vascular development and stability. Mural cells wrap around larger vessels and exert significant influence upon the regulation of blood flow, endothelial network growth, and vessel stability. However, little is know about the effect of their developmental origins or the signaling process that leads to vessel development. The development of vascular smooth muscle cells is a crucial target for vascular tissue engineering and therapeutic revascularization.[5]
Organ Systems Involved
Smooth muscle is present in all of the organ systems below:
- Gastrointestinal tract
- Cardiovascular - blood vessel and lymphatic vessels
- Renal - urinary bladder
- Genital - uterus, both male and female reproductive tracts
- Respiratory tract
- Integument - erector pili of the skin
- Sensory - the ciliary muscle and iris of the eye
Function
The primary function of smooth muscle is contraction. Smooth muscle consists of two types: single-unit and multi-unit. Single-unit smooth muscle consists of multiple cells connected through connexins that can become stimulated in a synchronous pattern from only one synaptic input. Connexins allow for cell-to-cell communication between groups of single-unit smooth muscle cells. This intercellular communication allows ions and molecules to diffuse between cells giving rise to calcium waves. This unique property of single-unit smooth muscle allows for synchronous contraction to occur.[6] Multi-unit smooth muscle differs from single-unit in that each smooth-muscle cell receives its own synaptic input, allowing for the multi-unit smooth muscle to have much finer control.
The function of smooth muscle can expand on a much larger scale to the organ systems it helps regulate. The functions of smooth muscle in each organ system is an incredibly broad topic and beyond the overall scope of this article. For simplicity, the basic functions of smooth muscle in the organ systems appear listed below.
- Gastrointestinal tract - propulsion of the food bolus
- Cardiovascular - regulation of blood flow and pressure via vascular resistance
- Renal - regulation of urine flow
- Genital - contractions during pregnancy, propulsion of sperm
- Respiratory tract - regulation of bronchiole diameter
- Integument - raises hair with erector pili muscle
- Sensory - dilation and constriction of the pupil as well as changing lens shape
Mechanism
Smooth muscle contraction depends on calcium influx. Calcium increases within the smooth muscle cell through two different processes. First, depolarization, hormones, or neurotransmitters cause calcium to enter the cell through L-type channels located in the caveolae of the membrane. Intracellular calcium then stimulates the release of calcium from the sarcoplasmic reticulum (SR) by way of ryanodine receptors and IP3; this process is referred to as calcium-induced calcium release.[7] Unlike skeletal muscle, smooth muscle calcium release from the sarcoplasmic reticulum does not physically couple to the ryanodine receptor. Once calcium has entered the cell, it is free to bind calmodulin, which transforms into activated calmodulin. Calmodulin then activates the enzyme myosin light chain kinase (MLCK), MLCK then phosphorylates a regulatory light chain on myosin. Once phosphorylation has occurred, a conformational change takes place in the myosin head; this increases myosin ATPase activity, which promotes interaction between the myosin head and actin. Cross-bridge cycling then occurs, generating tension. The tension generated is relative to the amount of calcium concentration within the cell. ATPase activity is much lower in smooth muscle than it is in skeletal muscle. This factor leads to a much slower cycling speed of smooth muscle. However, the more extended period of contraction leads to a potentially greater force of contraction in smooth muscle. Smooth muscle contraction is enhanced even further through the use of connexins. Connexins allow for intercellular communication by allowing calcium and other molecules to flow to neighboring smooth muscle cells. This action allows for rapid communication between cells and a smooth contraction pattern.
Steps involved in smooth muscle cell contraction:
- Depolarization of membrane or hormone/neurotransmitter activation
- L-type voltage-gated calcium channels open
- Calcium-induced calcium release from the SR
- Increased intracellular calcium
- Calmodulin binds calcium
- Myosin light chain kinase activation
- Phosphorylation of myosin light chain
- Increase myosin ATPase activity
- Myosin-P binds actin
- Cross-bridge cycling leads to muscle tone.
Dephosphorylation of myosin light chains terminates smooth muscle contraction. Unlike skeletal muscle, smooth muscle is phosphorylated during its activation, which creates a potential difficulty in that simply reducing calcium levels will not produce muscle relaxation. Myosin light chain phosphatase (MLCP), instead, is responsible for dephosphorylation of the myosin light chains, ultimately leading to smooth muscle relaxation.
Another important clinical aspect of smooth muscle relaxation is the mechanism of nitric oxide. Nitric oxide forms via nitric oxide synthase in endothelial cells; it is then able to diffuse out of the endothelium into smooth muscle cells. Nitric oxide then induces the conversion of guanosine triphosphate (GTP) to cyclic guanosine monophosphate (cGMP) by binding to and activating the enzyme guanylyl cyclase. In smooth muscle cells, the increase in cGMP will lead to stimulation of cGMP-dependent protein kinase, which in turn activates MLCP, leading to dephosphorylation of myosin light chains and eventual smooth muscle relaxation.
Smooth muscle action potentials are unique in that membrane potential acts to initiate or modulate contraction. As such, graded membrane response can become stimulated by multiple factors, including local humoral factors, circulating hormones, or mechanical stimulation like stretching of the cells. Action potentials in smooth muscle cells are slower than skeletal action potentials, and they can last almost fifty times as long. This characteristic appears to occur because calcium channels in smooth muscle cells open slower than skeletal muscle, which, in turn, leads to slow repolarization of smooth muscle as potassium channels are also slow to react. Sodium channels may also be present on the smooth muscle membrane and function by increasing the rate of depolarization and thus can aid in the activation of calcium channels.
Some smooth muscle cells also display the ability to form a spontaneous pacemaker current. This pacemaker current, for example, is maintained in the intestines by the interstitial cells of Cajal. The pacemaker current represents repetitive oscillations in the membrane potential that occur in several cycles. These slow waves of membrane potential fluctuation are unique in that they are not responsible for the contraction of the intestines. It appears that at resting membrane potential, some voltage-gated calcium channels become active, an influx of calcium will then propagate a slow wave at a specific threshold. If the amplitude of the slow-wave is high enough, L-type calcium channels will open, leading to contraction.[7] Sodium may also play a role in the oscillating electrical activity. Calcium influx stimulates Na-Ca exchange, which leads to an influx of sodium; this will effectively increase the rate of the Na-K pump. This activity all remains unique because the oscillations of the membrane potential and slow-wave activity generates without the influence of the central nervous system. The slow waves are, therefore, able to allow the smooth muscle to remain tonic without having to maintain continuous action potential firings.
Research has shown that smooth muscle can contract without any action potential. In multi-unit smooth muscle, action potentials usually do not occur. An example would be the smooth muscle in the iris of the eye where norepinephrine and ACh generate a depolarization that is called a junctional potential. In these situations, the neurotransmitters themselves create the changes in the smooth muscle to cause the contraction. The junctional potential eventually triggers an influx of calcium through L-type channels. In some situations, the neurotransmitters may activate a G-protein, which activates phospholipase C generating IP3; IP3 is then able to trigger calcium release from the sarcoplasmic reticulum.[8]
Smooth muscle contractions may be required to last for a long time. The metabolic demand of sustained contraction would be far too costly if smooth muscle contractions occurred similarly to skeletal muscle. The muscle would most likely fatigue as intracellular supplies of ATP become depleted. The mechanism that allows the smooth muscle to maintain high-tension at low energy consumption; termed the latch state.[9] Even as levels of phosphorylated myosin light chain kinase decrease, smooth muscle tone will remain high.
Related Testing
Anti-smooth muscle antibodies (ASMA) are serum markers that can become elevated in some forms of auto-immune disease. They are typically associated with auto-immune hepatitis but may also increase in primary sclerosing cholangitis and systemic lupus erythematosus. The antibodies direct themselves against actin, troponin, and tropomyosin.
Pathophysiology
The pathophysiology of smooth muscle is incredibly diverse, and the limited scope of this article will not be able to cover it in detail. A brief description of how different organ systems may be affected by smooth muscle pathologies is listed below. Much of the material covered is also applicable to other diseases not mentioned in this article. As a clinician, it is more important to be able to recognize when smooth muscle may play a role in the disease process.
The gastrointestinal tract is mostly dependent on smooth muscle for motility. Any damage to the smooth muscle of the intestines may have a devastating effect on the body. The term for this loss of motility is gastroparesis. Many conditions can impact gastric motility, including nerve dysfunction, collagen disease, muscular dystrophies, amyloidosis, thyroid disease, diabetes mellitus, Chagas disease, neuropathy, and so on. A spectrum of disease can occur in these patients; they may present asymptomatic, or they may present in crisis with functional gastric obstruction.[10] Gastric disorders should always immediately raise suspicion that there are potential impacts to smooth muscle physiology.
In the renal system, vascular smooth muscle is present in the kidneys, throughout the ureters, and in the bladder. At the level of the kidneys, vascular smooth muscle dysfunction is associated with chronic kidney disease and can lead to end-stage renal disease.[11] Damage to the ureters may also damage smooth muscle and impair ureter function, as in the case of nephrolithiasis. The functionality of the bladder relies almost exclusively on the unique properties of smooth muscle. Damage to any of the systems that regulate smooth muscle in the bladder can lead to loss of tone and subsequent neurogenic bladder disease; this becomes more complex when you consider the effects such a disease has on a person's quality of life.[12]
In the genital system, smooth muscle is often a focus regarding its role in childbirth. Smooth muscle lines the uterus, which creates the contractile force during childbirth. Many pharmaceuticals exist specifically to help enhance smooth muscle contraction at the time of birth. While this may not represent an actual pathology, it is crucial to recognize that physicians can use a knowledge of smooth muscle physiology to prevent pathologies from occurring. In males, fertility is also a function of the contractions of smooth muscle in the epididymis and vas deferens. Without the contractile nature of smooth muscle, spermatozoa would never be able to assist in fertilization; this becomes important because of the apparent lack of information on the possible pathologic effects of smooth muscle and infertility. For example, many medications that are frequently used by males impact smooth muscle contractility and, therefore, may also affect fertility. Examples include nonsteroidal anti-inflammatory drugs, phosphodiesterase (PDE) inhibitors, nitrates, adrenergic receptor antagonists and agonists, psychotropic drugs, anticholinergic drugs, calcium antagonists, and ace inhibitors.[13]
Perhaps the most well know the pathophysiology of smooth muscle occurs in the cardiovascular and respiratory systems. Within the cardiovascular system, smooth muscle helps to regulate blood flow by controlling the diameter of the vessel. As previously discussed, vascular pathologies of smooth muscle can have devasting effects on the body and lead to significant pathology. Atherosclerosis, once thought to be only a function of hemodynamics and vessel structure, has more recently been linked to smooth muscle development.[4] Research has even shown that continuous vascular smooth muscle activation can lead to the formation of pulmonary hypertension.[14] Within the lungs, pathologic activation of smooth muscle can lead to the development of asthma. Asthma occurs when smooth muscle constriction leads to obstruction of the airway. Recent studies have shown that the smooth muscle layer may increase in thickness before the onset of asthma even occurs, pointing towards a potential genetic link.[15]
Clinical Significance
Estimates are that in the year 2013, health care costs associated with asthma reached $81.9 billion in the United States.[16] With such a significant health care burden, it is astonishing to realize that asthma results from something as simple as smooth muscle contraction. Smooth muscle is an integral part of the human body; its function is required for life and is present in almost every organ system. In the cardiovascular system, smooth muscle is used in vessels to maintain blood pressure and flow; in the lungs, it opens and closes airways; in the gastrointestinal system it plays a role in motility and nutrition collection; and yet it still serves a purpose in almost every other organ system as well. The wide distribution of smooth muscle throughout the body and its many unique properties make it imperative for medical professionals to have an in-depth understanding of its physiology, function, and disease applications.
From a functional aspect, smooth muscle physiology is responsible for maintaining and preserving every vital sign. Regardless of whether a patient presents with acute emergent disease or a chronic disease, it is likely that smooth muscle has played some role in its development. In an acute setting, many life-saving therapies directly target smooth muscle. In these settings, a firm foundation and understanding of smooth muscle will help health professionals save lives. An even broader understanding of smooth muscle will assist clinicians in increasing the quality of life of their patients. As part of the biopsychosocial model, it is also important to take into consideration the psycho-social factors that may be overlooked with the diseases of smooth muscle; for example, a patient diagnosed with neurogenic bladder disease may become socially isolated to avoid the embarrassment associated with their disease state. When approaching smooth muscle dysfunction, healthcare providers need to appreciate the many facets of how the disease will impact their patients.
As with all aspects of medicine, a continuing amount of research will likely change our future understanding of smooth muscle and its overall effects on disease. Current research into smooth muscle has shown promise in future implications, such as restoring endothelial tissue, which in the future could point to new ways to encourage revascularization. Even small changes in understanding like this could have an immeasurable impact on the treatment and mortality of cardiovascular disease in the future.[4] While smooth muscle physiology remains an exceptionally deep topic, a solid understanding of its impact on healthcare, even at the most basic level, will give healthcare professionals tools to provide better healthcare outcomes now and into the future.