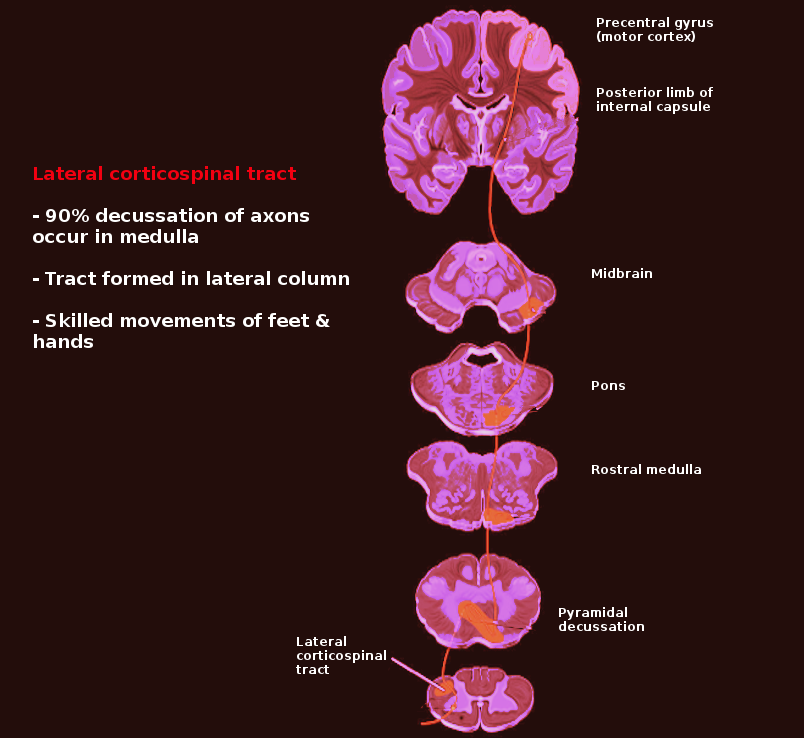
[1]
Rong D, Zhang M, Ma Q, Lu J, Li K. Corticospinal tract change during motor recovery in patients with medulla infarct: a diffusion tensor imaging study. BioMed research international. 2014:2014():524096. doi: 10.1155/2014/524096. Epub 2014 May 25
[PubMed PMID: 24967374]
[2]
Jang SH. The corticospinal tract from the viewpoint of brain rehabilitation. Journal of rehabilitation medicine. 2014 Mar:46(3):193-9. doi: 10.2340/16501977-1782. Epub
[PubMed PMID: 24531325]
[3]
Welniarz Q, Dusart I, Roze E. The corticospinal tract: Evolution, development, and human disorders. Developmental neurobiology. 2017 Jul:77(7):810-829. doi: 10.1002/dneu.22455. Epub 2016 Oct 14
[PubMed PMID: 27706924]
[4]
Seo JP, Jang SH. Characteristics of corticospinal tract area according to pontine level. Yonsei medical journal. 2013 May 1:54(3):785-7. doi: 10.3349/ymj.2013.54.3.785. Epub
[PubMed PMID: 23549830]
[5]
Kamson DO, Juhász C, Shin J, Behen ME, Guy WC, Chugani HT, Jeong JW. Patterns of structural reorganization of the corticospinal tract in children with Sturge-Weber syndrome. Pediatric neurology. 2014 Apr:50(4):337-42. doi: 10.1016/j.pediatrneurol.2013.12.012. Epub 2013 Dec 18
[PubMed PMID: 24507695]
[6]
Hong JH, Son SM, Byun WM, Jang HW, Ahn SH, Jang SH. Aberrant pyramidal tract in medial lemniscus of brainstem in the human brain. Neuroreport. 2009 May 6:20(7):695-7
[PubMed PMID: 19402216]
[7]
Kamiyama T, Kameda H, Murabe N, Fukuda S, Yoshioka N, Mizukami H, Ozawa K, Sakurai M. Corticospinal tract development and spinal cord innervation differ between cervical and lumbar targets. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2015 Jan 21:35(3):1181-91. doi: 10.1523/JNEUROSCI.2842-13.2015. Epub
[PubMed PMID: 25609632]
[8]
Pangelinan MM, Leonard G, Perron M, Pike GB, Richer L, Veillette S, Pausova Z, Paus T. Puberty and testosterone shape the corticospinal tract during male adolescence. Brain structure & function. 2016 Mar:221(2):1083-94. doi: 10.1007/s00429-014-0956-9. Epub 2014 Dec 11
[PubMed PMID: 25503450]
[9]
Zhou Y, Zhang R, Zhang S, Yan S, Wang Z, Campbell BCV, Liebeskind DS, Lou M. Impact of perfusion lesion in corticospinal tract on response to reperfusion. European radiology. 2017 Dec:27(12):5280-5289. doi: 10.1007/s00330-017-4868-y. Epub 2017 May 24
[PubMed PMID: 28540481]
[10]
Moses ZB, Abd-El-Barr MM, Chi JH. Timing is everything in corticospinal tract recovery after stroke. Neurosurgery. 2015 Apr:76(4):N18-9. doi: 10.1227/01.neu.0000462697.23265.f4. Epub
[PubMed PMID: 25784011]
[11]
Spampinato MV, Kocher MR, Jensen JH, Helpern JA, Collins HR, Hatch NU. Diffusional Kurtosis Imaging of the Corticospinal Tract in Multiple Sclerosis: Association with Neurologic Disability. AJNR. American journal of neuroradiology. 2017 Aug:38(8):1494-1500. doi: 10.3174/ajnr.A5225. Epub 2017 Jun 1
[PubMed PMID: 28572153]
[12]
Manogaran P, Vavasour I, Borich M, Kolind SH, Lange AP, Rauscher A, Boyd L, Li DK, Traboulsee A. Corticospinal tract integrity measured using transcranial magnetic stimulation and magnetic resonance imaging in neuromyelitis optica and multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England). 2016 Jan:22(1):43-50. doi: 10.1177/1352458515579441. Epub 2015 May 6
[PubMed PMID: 25948623]