Continuing Education Activity
Corneal transplantation, a pivotal intervention for various corneal disorders, involves replacing damaged or diseased corneal tissue with a healthy donor cornea. The goal of the procedure is to restore visual function, addressing conditions such as keratoconus, Fuchs endothelial dystrophy, and corneal scars. Techniques have evolved from full-thickness transplants to selective lamellar procedures like Descemet stripping endothelial keratoplasty (DSEK) and Descemet membrane endothelial keratoplasty (DMEK), enhancing outcomes and reducing complications. This continuing education activity thoroughly explores corneal transplantation, encompassing anatomy, historical context, indications, contraindications, surgical techniques, and recent advancements.
Clinicians engaging with this activity will gain comprehensive insights into the evolving landscape of corneal transplantation. From understanding the historical progression to mastering modern techniques such as DSEK and DMEK, clinicians enhance their competence in patient assessment, surgical decision-making, and postoperative care. The activity emphasizes the importance of effective communication with patients and collaboration within a multidisciplinary healthcare team. Clinicians are equipped with the latest knowledge on advancements in immunology, tissue banking, and surgical approaches, ensuring they stay at the forefront to provide optimal care to patients undergoing corneal transplantation.
Objectives:
Identify potential candidates for corneal transplantation by conducting comprehensive evaluations, considering factors such as the nature of corneal disorders, patient history, and suitability for various transplantation techniques.
Apply a comprehensive understanding of corneal anatomy, history, indications, contraindications, personnel, equipment, techniques, and complications to enhance decision-making and surgical proficiency.
Assess postoperative outcomes and complications, monitoring patients for signs of rejection, infection, or other adverse events, and adjusting treatment plans accordingly.
Collaborate with a multidisciplinary healthcare team, including ophthalmic surgeons, advanced practitioners, nurses, pharmacists, and other professionals, to ensure coordinated and patient-centered care throughout the transplantation process.
Introduction
Corneal disease is the fifth leading cause of global blindness, necessitating interventions such as keratoplasty, the most prevalent and successful human transplantation.[1] The first successful transplant was completed in 1905.[1][2] Various dystrophic, infectious, degenerative, and inflammatory corneal disorders, often secondary to ocular surface diseases, contribute to corneal blindness.[3] The widespread and intricate epidemiology involves infectious and nutritional disorders like trachoma, xerophthalmia, river blindness, and microbial keratitis, ranking second to cataracts in ocular conditions causing blindness globally. Developing countries, particularly in Africa and Asia, experience higher incidences, and corneal scarring remains a leading cause of reversible blindness in children.[4] Despite corneal transplantation being the primary visual rehabilitation method, its efficacy is constrained by limited expertise and donor corneal tissue availability, particularly in these developing nations.[5]
In the Western world, inherited, degenerative, and iatrogenic conditions like Fuchs corneal endothelial dystrophy, keratoconus, and pseudophakic bullous keratopathy (PBK) are more prevalent, with a favorable prognosis. Corneal transplantation, a globally widespread procedure, addresses diverse indications such as keratoconus, PBK, corneal scars, dystrophies, and microbial keratitis.[6] However, in developing nations, the demand for treatment often outstrips the available supply. A previous article reported that in 2010, the number of corneal transplants in the United States was 42,642; in 2008, there were 12,623 solid organ transplants.[7][8] In countries like India, where the incidence of microbial keratitis is high, there were 27,075 corneal transplants performed between April 2019 and March 2020. However, due to the COVID-19 pandemic, the number of transplants fell to 12,998 performed between April 2020 and March 2021, as per data from the Eye Bank Association of India. The demand for corneal grafts often exceeds the supply in most eye banks, especially in developing countries, resulting in long waiting periods. This activity provides a comprehensive understanding of corneal transplantation, covering anatomy, history, indications, contraindications, personnel, equipment, techniques, and complications, offering valuable insights for students and clinicians.[9]
History of Corneal Transplantation
The history of corneal transplant traces its roots to 1800, but significant strides occurred in the past 2 decades with the introduction of lamellar transplantation techniques. The evolution of modern corneal transplantation results from centuries of ideas, experimentation with xenografts and allografts, and resilience.[10] As early as 1789, French surgeon Pellier de Quengsy proposed using a transparent material to replace an opaque cornea.[11] The first documented report of anterior lamellar corneal transplantation dates back to 1800. Karl Himley, in 1813, envisioned and experimented with xenografts, with his student Franz Reisinger attempting the first experimental xenograft transplantation in 1824, albeit without success.
In 1838, Richard Kissam achieved a milestone by performing the first therapeutic corneal xenograft using a porcine cornea. The first successful human allograft and penetrating keratoplasty (PKP), following anesthetics and antiseptic surgery developments, was performed by Eduard Zirm in December 1905 on a farm laborer with lime burns.[12][13] Lamellar corneal transplants emerged as a pivotal advancement, mitigating the risk of endothelial graft failure. The introduction of topical steroids and refined surgical techniques in PKP in 1950 set the gold standard for corneal transplantation. The era of lamellar transplantation began in 2005, revolutionizing keratoplasty techniques. Successful outcomes hinge on meticulous ocular environment preparation, addressing inflammatory factors, and comprehensive systemic control, especially in cases involving rheumatoid arthritis and systemic lupus erythematosus. Preoperative control of diabetes, hypertension, cardiac, respiratory, and renal disease is also mandatory in each case.[14]
Recent Developments
Advancements in immunology, surgical techniques, and tissue banking have greatly influenced the field of corneal transplantation.[15][16] Over the last 2 decades, significant developments in selective endothelial replacement techniques have led to notable changes in the field. Gerrit Melles introduced a posterior lamellar keratoplasty (PLK) that involved transplanting only a portion of the cornea. The procedure involved making an incision at the limbus and dissecting and replacing the endothelium, the Descemet membrane (DM), and the posterior stroma with a donor button consisting of the same corneal layers. The donor button was held in place by an air bubble.[17]
Mark Terry made modifications to the PLK procedure in 2001. He used viscoelastic material instead of an air bubble and renamed deep lamellar endothelial keratoplasty (DLEK).[18] In 2004, Gerrit Melles modified the technique by removing only the host endothelium and DM, thus eliminating the need for stromal dissection. He replaced it with a donor button of the endothelium, DM, and stroma, creating Descemet stripping endothelial keratoplasty (DSEK). This technique was later automated using a microtome, resulting in Descemet stripping automated endothelial keratoplasty (DSAEK).[19][20][21] In 2006, the technique was further developed by transplanting only a donor button of endothelium and DM without the posterior stroma, creating DM endothelial keratoplasty (DMEK).[21] Minor modifications include automated posterior lamella dissection similar to DSEK or DM automated endothelial keratoplasty (DMAEK).[22]
A selective keratoplasty technique related to the anterior cornea is the deep anterior lamellar keratoplasty (DALK), whereby a donor button replaces the epithelium, Bowman layer, and stroma.[23] This selective lamellar transplant involves replacing only the diseased layer of the cornea while retaining the healthy layers, resulting in better visual outcomes and reducing the complication rate. The cornea has 5 layers, and only the Bowman layer, stroma, DM, and endothelium can be replaced.[24] The corneal endothelial cells have poor regenerating capacity, and any loss of these cells due to trauma or disease can lead to corneal edema. In contrast, PKP transplants all 5 layers of the cornea. In the United States, endothelial transplant rates increased significantly from 5% in 2005 to 44.9% in 2010. In 2022, the number of PKPs, DSAEK, and DMEK was almost equal, around 15,000 each. Lamellar transplant is now preferred over PKP for various indications, such as keratoconus, Fuchs endothelial dystrophy, and PBK.[25]
Anatomy and Physiology
Macroanatomy
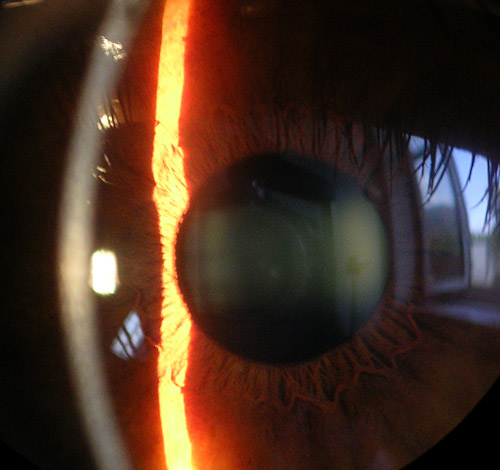
The cornea, a transparent avascular structure, plays a vital role in safeguarding the internal components of the eye and contributes significantly to two-thirds of its refractive power. The convex cornea, positioned as the foremost part of the eyeball, has a diameter of about 11.5 mm; the thickness varies from the center, measuring 550 to 565 μm, to the periphery, ranging from 610 to 640 μm.[26] The cornea is richly innervated by the extensive network of long ciliary nerves originating from the ophthalmic division of the trigeminal nerve (see Image. Cornea, Iris, and Lens, Slit Lamp Image).
Microanatomy
The corneal layers, arranged anteroposteriorly, include:
- Epithelium [27]
- This serves as the primary barrier to the eye, offering a smooth optical surface, and fulfills immunological functions. Comprising 5 to 7 cell layers at the center and 7 to 10 cell layers at the periphery, it maintains an approximate thickness of 50 μm. According to the limbal stem cell hypothesis, stem cells and transient amplifying cells proliferate in the periphery, migrating centrally to replace surface cells.[28] The epithelium comprises squamous cells, wing cells, and basal cells.
- Bowman layer
- This layer of resilient tissue primarily comprises collagen types I and V, which act as an acellular condensate of the stroma, influencing the corneal shape. Notably, this layer exhibits regenerative properties.
- Stroma
- The stroma provides mechanical strength and refractive power, featuring regularly arranged collagen fibers, glycosaminoglycans, and interconnected keratocytes in packed layers or lamellae. At the corneal center, 200 lamellae are present, with a higher packing density and interconnectivity in the anterior region than in the posterior. In procedures like DSEK and DSAEK, introducing an air bubble to separate the endothelium and DM results in the adherence of part of the posterior stroma to the DM, suggesting a natural cleavage plane 10 μm above the DM.[29] This specific region of the posterior stroma has been termed the pre-Descemet or Dua layer.[30]
- Dua layer
- This layer is 10 to 15 μm thick and firmly adheres to the stromal fibers.
- DM
- This 7- to 10-micrometer structure primarily comprises type IV collagen and laminin, continuously secreted by endothelial cells.[31] The DM is a base for endothelial cells, contributing significantly to corneal transparency.
- Endothelium
- This simple cuboidal monolayer rich in mitochondria is crucial in maintaining transparency.
Indications
DALK stands out as the preferred surgical intervention for patients presenting with a posterior corneal scar and a healthy endothelium.[24] In instances marked by endothelial decompensation and the presence of an anterior stromal scar, full-thickness PKP emerges as the surgery of choice. However, when the anterior stroma remains unaffected, DSEK and DSAEK are the preferred surgical options. Corneal transplants are categorized based on cosmetic, tectonic, optical, and therapeutic indications. Opting for a lamellar transplant brings several advantages, including a reduced risk of expulsive choroidal hemorrhage, preserved anatomical integrity, and a lower incidence of graft rejection in the postoperative period due to minimal tissue manipulation and a smaller amount of transplanted tissue.[32]
Corneal blindness remains a pervasive global issue, with the indications for corneal transplants varying across regions. In developed countries, bullous keratopathy is the most prevalent reason for corneal transplantation. In contrast, infective keratitis and corneal scars are more common indications in developing countries.[6] Notably, there has been a transformative trend in developing nations concerning corneal transplantation, where the landscape has shifted from PBK to corneal degenerations causing blindness.
An assessment by Park et al on the international distribution of corneal grafts by the Eye Bank Association of America revealed a substantial increase in graft numbers, from less than 1,000 in 2005 to 24,400 in 2014. The primary indications for corneal transplants include PBK, keratoconus, Fuchs endothelial dystrophy, and failed grafts. Interestingly, the scenario has evolved, with PBK transitioning from being the most common indication before 2005 to the third most common by 2014, surpassed by Fuchs and keratoconus.[33]
Moreover, an increasing number of PKPs are now being performed for congenital opacities like Peter anomaly, congenital hereditary endothelial dystrophy (CHED), sclerocornea, and corneal dermoid. On the other hand, lamellar keratoplasty is commonly indicated for degenerations, peripheral ulcerative keratitis (PUK), keratoconus, and nonspecific anterior lamellar stromal scarring. In keratoconus treatment, DALK has become more prevalent than traditional PKP.[34]
The possible reasons for performing a corneal transplant, also known as PKP, include conditions such as keratoconus, ectasias, corneal degenerations, dystrophies, keratitis, congenital opacities, chemical or mechanical corneal trauma, and regrafts. However, the leading indications for PKP may vary depending on a region's sociopolitical, geographical, and economic factors and may not accurately represent the most common causes of corneal pathology.[35]
Superficial corneal scars can be caused by various factors such as healed keratitis, trachoma, trauma, and corneal dystrophies that affect the anterior stroma and degenerations. Endothelial keratoplasty (EK) is a surgical procedure that includes different types of EK procedures, such as PLK, DLEK, DSEK, DSAEK, DMEK, and DMAEK. It is important to note that EK procedures are not suitable for patients with healthy corneal endothelium and are only recommended for patients with specific conditions such as keratoconus, Fuchs endothelial dystrophy, posterior polymorphous dystrophy, CHED, bullous keratopathy, iridocorneal endothelial syndrome, and failed EKs.[36] DALK is another surgical procedure that focuses on the anterior cornea (epithelium, Bowman layer, and stroma) and is recommended for patients with keratoconus and corneal scars. The indications for DALK include deep stromal scars caused by healed infective keratitis, healed chemical injuries with deep stromal scars, keratoconus, and stromal dystrophies.[37]
Contraindications
The absolute and relative contraindications for corneal transplantation differ from country to country and are region-dependent.
In the United Kingdom, the National Health Services Blood and Transplant Agency lists the following as absolute contraindications:
- The transplant is unlikely to restore corneal function or integrity.
- The transplant fails to remove tissue that would otherwise lead to further damage to the eye.[38]
Equipment
Basic operating room equipment and the ability to use local anesthesia (eg, retrobulbar block) in addition to intravenous sedation or general anesthesia are required.[39]
Personnel
The team responsible for the procedure includes the surgeon performing the operation, an assistant, an anesthesiologist and nursing personnel.
Preparation
The procedural details must be clearly explained to the patient, allowing ample time for understanding and evaluating the associated risks and benefits to ensure a thorough and transparent communication process. Subsequently, informed consent is obtained. Before the procedure, a comprehensive history, encompassing current medications and allergies, is taken, and an ophthalmic examination is conducted.[40] Given that a significant portion of the patient population suitable for this procedure may be older with comorbid conditions, it is imperative to provide tailored advice regarding their medications (ie, blood thinners, antihypertensives, diabetic medications). Many healthcare organizations have established guidelines, protocols, or instructions concerning managing such medications, advising patients on whether to discontinue or continue them preoperatively.
Clear communication with the patient or their caregivers is necessary for early identification of complications and time management.[41] Patients should be provided with contact numbers for immediate assistance, considering potential postoperative issues. Additionally, arrangements for transportation and care following the procedure should be made in collaboration with a designated next of kin, recognizing the importance of a supportive postoperative environment.[42] This holistic approach ensures not only procedural success but also the comprehensive well-being and support of the patient throughout the entire process.
Technique or Treatment
PKP
After administering anesthesia, an eyelid speculum is delicately inserted, and the patient's eye is secured, employing either a scleral fixation ring or sutures. Precision is used in measuring the host corneal diameter to determine the fitting size for the donor button. The donor button is trephinated to achieve dimensions 0.25 to 0.5 mm larger than the host trephination.[43] Placing a trephine on the host's cornea, a button is excised three-quarters through the stroma, accompanied by a peripheral iridotomy/iridectomy. The secure attachment of the donor button to the host corneal tissue is achieved through the application of nonabsorbable sutures meticulously rotated to bury the knots. Viscoelastic hyaluronic acid may prevent the iris from adhering to the suture line if necessary. Following the procedure, antibiotic therapy is administered in adherence to local protocols, and the eye is appropriately shielded.
The advent of advanced anterior lamellar keratoplasty (ALK) and EK techniques has demonstrated comparable or superior visual outcomes for patients, coupled with reduced complication rates.[43] Despite the evolving preference for lamellar keratoplasty techniques, PKP remains the globally recognized gold standard. In cases involving deeper corneal scars devoid of inflammation, such as hydrops, keratoconus, infection, and trauma, PKP consistently delivers favorable results. In instances of microbial keratitis, therapeutic keratoplasty remains the primary technique, yielding excellent outcomes. However, DALK has emerged as a successful alternative, particularly in treating keratoconus, replacing traditional PKP with positive results.[44]
ALK
The inception of ALK traces back to 1948 when Paufique et al reintroduced the technique. In 1964, Barraquer made a groundbreaking contribution by describing and applying the microkeratome to treat high refractive errors through keratomileusis and keratophakia. The microkeratome's precision in generating regular cuts proved pivotal, mitigating the risk of poor visual outcomes associated with irregular surfaces. Kauffman later introduced epikeratophakia, a lamellar grafting technique without necessitating host corneal dissection.[45]
ALK can be accomplished through various methods, including manual dissection, microkeratome, and femtosecond laser-assisted dissection. This procedure entails replacing the diseased anterior stroma of the host with donor corneal tissue while preserving the unaffected Descemet membrane and endothelium. This approach minimizes the risk of endothelial graft rejection, a common cause of post-PKP graft failure. However, mastering ALK presents technical challenges, requiring a steep learning curve due to the intricate manual layer-by-layer dissection of the anterior corneal layer from the DM and endothelium.[46]
Descemetic DALK involves the removal of the entire corneal stroma with minimal impact on the host endothelium. This technique circumvents the stroma-to-stroma interface, which may compromise visual acuity in ALK and predescemetic DALK. The popular big bubble technique described by Anwar is often employed in DALK, where a deep stromal air bubble forms, separating the DM from the stroma by creating a substantial bubble between the 2 layers. Despite its efficacy, DALK is not without complications, with common issues including microperforation and macroperforation of the DM, double anterior chamber, and interface irregularities. The reported perforation rates range from 4% to 39% in various series, and the conversion rate to PKP has been documented to be 0% to 14% in previous reports.[24]
Superficial Anterior Lamellar Keratoplasty (SALK)
In 2003, Kaufman et al described a technique called SALK that treats superficial corneal opacities up to 30% to 40% of the cornea. This method is sutureless and utilizes a lamellar donor graft of 200 um that is fixed with fibrin glue.[47]
Automated Lamellar Therapeutic Keratoplasty (ALTK)
This technique is recommended for treating corneal opacities in the cornea's front to middle layers. During the procedure, a microkeratome separates the host and donor corneas, resulting in a better fit and improved interface between the 2.[48]
DALK
Two types of deep anterior corneal opacity can be treated with DALK: predescemetic DALK and descemetic DALK. Predescemetic DALK is recommended for patients with thin corneas or a high risk of perforation.[24] This technique aims to remove the pathology while leaving a small amount of posterior stroma and endothelium intact. In descemetic DALK, the complete corneal stroma is removed, while the bare DM is left behind. DALK can be performed using various techniques, such as manual dissection and deeper dissection with air or viscoelastic, as defined by Archila and Melles. Anwar and Teichmann developed the big bubble technique of DALK dissection, which involves using air to dissect the deeper stroma and expose the DM.[24]
PLK
PLK focuses on replacing the diseased endothelium with a healthy donor graft while the remainder of the cornea remains untouched. Common indications for PLK include conditions such as Fuchs endothelial dystrophy, posterior polymorphous corneal dystrophy, PBK, CHED, iridocorneal endothelial syndrome, viral endothelitis with corneal decompensation, and aphakic bullous keratopathy.[49]
The early approaches to PLK involved anterior dissection to replace the posterior corneal lamella, but this method did not gain popularity. In 1956, Tillet introduced the posterior lamellar approach; however, the visual outcomes and graft survival were suboptimal due to manual dissection and sutures.[3] Melles et al revolutionized the technique by employing air tamponade instead of sutures for graft attachment. The posterior stromal lenticule was dissected and replaced with a donor button through a 9 mm limbal incision. PLK, as first introduced by Melles et al, involved folding a donor button and inserting it through a 5 mm incision. Terry and Ousley referred to this in the USA as DLEK.[17]
Melles et al further advanced the technique and introduced the concept of Descemet stripping endothelial keratoplasty (DSEK). This innovation led to Descemet stripping of descemetorhexis, where the DM and endothelium are removed by stripping and replaced with a lenticule from the donor tissue. When the same technique was executed using an automated microkeratome for donor tissue dissection, as described by Gorovoy, it was labeled as DSAEK. This modification reduced the complexity of the surgery, resulting in a smoother interface, improved learning curves, and enhanced visual outcomes.
A groundbreaking advancement in 2006 by Melles et al involved replacing only the diseased endothelium with healthy endothelium, giving rise to DMEK.[50] Despite its superior visual outcomes, DMEK faced limited acceptance due to technical intricacies and surgical challenges. EK, encompassing DSEK and DMEK, is now widely regarded as a superior and more popular alternative to PKP. This preference is attributed to the avoidance of catastrophic complications such as expulsive choroidal hemorrhage and choroidal detachment associated with open globe procedures. Additionally, EK ensures early visual recovery, sidestepping suture-related complications and mitigating astigmatism. Owing to their earlier and superior visual outcomes, DSEK and DMEK are now considered akin to refractive surgery.[51]
DMEK
Upon the induction of anesthesia, the procedure commences with the insertion of an eyelid speculum, ensuring the patient's eye is steadfastly secured using either a scleral fixation ring or sutures. Creating 2 to 4 paracentesis sites and a temporal corneal incision, the anterior chamber is filled with viscoelastic hyaluronic acid.[51][52] A peripheral iridotomy is meticulously fashioned to prevent pupillary block. Employing the donor's corneal epithelium with a circular ring, a resection template is created, and the DM is peripherally scored, peeled from the overlying stroma, and subsequently removed. The donor button is stained with a trypan blue solution to ensure visibility and carefully inserted into the anterior chamber. The injection of an air or sulfur hexafluoride bubble guarantees adhesion. Closure of the primary incision is achieved with a buried, nonabsorbable suture. Antibiotic therapy is administered following local protocols, and the eye is shielded.
EK has emerged as the preferred treatment for endothelial disorders, almost entirely supplanting full-thickness procedures. Two prominent techniques in this realm are DSEK and DSAEK. In DSEK, the meticulous dissection of the lenticule is carried out manually. In contrast, DSAEK involves the insertion of a precut lenticule into the cornea, representing significant advancements in the field.[53]
Paradigm Shift: A Change From Full Thickness to Lamellar Transplant
In a comprehensive review conducted by Darlington et al, spanning from 1980 to 2004, a substantial transformation in corneal transplantation trends was noted. Notably, more than 95% of corneal tissues were employed in PKP, with primary indications being Fuchs endothelial dystrophy, keratoconus, PBK, and failed grafts.[54] Rock et al, in a 12-year evaluation of corneal transplantation trends, observed a doubling in the mean rate of corneal transplantation from 71 per year in the initial 6 years, which then increased to 139% in the subsequent 6 years. A remarkable shift from full-thickness transplants to lamellar procedures, such as DSEK and DMEK, was evident during this period.[55]
Zhang et al further investigated the changing trends in keratoplasty from 2000 to 2012, noting a significant decrease in PKP procedures and an increase in DALK and DSAEK since the introduction of lamellar transplants in 2006. In managing Fuchs endothelial dystrophy cases, DSAEK became the preferred choice for approximately 80% of patients from 2011 to 2012.[56] A study by Le et al at the University of Toronto revealed that 61% of keratoplasties were lamellar procedures, with DSAEK being the most common, followed by DALK and DMEK. In contrast, 39% of cases involved full-thickness PKP.
Park et al, analyzing corneal transplantation trends from 2005 to 2014, reported a significant reduction in the rate of PKP (85% to 42%) and a substantial increase in lamellar transplants (5% to 85%). DSAEK emerged as the most common corneal transplant procedure in the USA by 2014. Notably, since 2011, the annual count of DMEK cases has risen, doubling by 2014. Approximately 75% of cases involving corneal decompensation were managed by EK in 2014, making it the preferred modality for 90% of Fuchs endothelial dystrophy cases and 60% of PBK cases. PKP, on the other hand, remained the treatment for about 40% of PBK cases.[57]
Zara et al, over a 6-year study period, identified PKP as the most common surgical technique, followed by DALK and DSAEK. However, during their analysis period, there was a notable decrease in PKP cases and a corresponding increase in lamellar transplants.[58]
Complications
Complications following surgery can be categorized into 2 types: early and late. Early complications can occur within days to weeks after the operation and include wound leakage, raised intraocular pressure, bleeding, and infection. Late complications, on the other hand, can occur months to years after the operation and may include:
- Corneal swelling
- Cataracts
- Graft failure
- Graft dislocation
- Graft rejection
- Pupillary block glaucoma
- Graft infection
- Graft infiltrates
- Endophthalmitis
- Panophthalmitis
- Epithelial ingrowth
- Double anterior chamber
- Eccentric graft
- Astigmatism
- DM detachment
- DM perforation
- Secondary glaucoma
- Lens expulsion
- Expulsive choroidal hemorrhage
- Recurrence of the original disease
The reported 5-year and 15-year graft survival rates are approximately 70% and 50%, respectively.[59][60]
Clinical Significance
The inception of successful corneal transplants dates back more than 110 years, marking it as one of the most common transplant procedures globally today. The US stands at the forefront with the highest rates of corneal transplants, witnessing a remarkable surge in EK over PKP, constituting approximately 60% of all transplants.[61] However, this trend is distinct, as a third of countries worldwide report no completed EKs, making the proportion considerably lower beyond the borders of the US.[61]
The landscape of corneal transplantation has undergone a transformative shift with the introduction of selective keratoplasty techniques, significantly enhancing graft survival, refractive outcomes, and visual acuity.[62] These advancements have, in turn, lowered the threshold for surgical intervention, fostering a heightened demand for human cadaveric corneas. Remarkably, only a modest 30% to 40% gap exists between the available corneas and the escalating demand.
An inherent challenge is that corneal endothelial cells (CECs) resist expansion post-biopsy in laboratory conditions. Recent research has explored alternative stem cells, including mesenchymal stem cells and induced pluripotent stem cells directed towards a neural crest and endothelial lineage through rho-associated protein kinase inhibition.[63][64] Large animal models have demonstrated promising results in generating CECs in vitro.[65] Upon further optimization, this approach may emerge as a therapeutic avenue for endothelial replacement, particularly in cases of CEC dysfunction, such as in Fuchs endothelial corneal dystrophy. The ongoing exploration of these regenerative strategies holds the potential to redefine the landscape of corneal transplantation, offering innovative solutions for challenging clinical scenarios.
Enhancing Healthcare Team Outcomes
A multidisciplinary team comprising physicians, advanced practitioners, nurses, pharmacists, and other health professionals ensures comprehensive and patient-centered care in corneal transplantation. Physicians, particularly ophthalmic surgeons specializing in corneal procedures, bring their surgical expertise and decision-making skills to the forefront. Advanced practitioners, such as physician assistants or nurse practitioners, contribute by conducting pre- and postoperative assessments, collaborating closely with physicians, and providing valuable patient education.
Nurses are integral to care coordination, preoperative preparations, postoperative monitoring, and patient follow-up. Pharmacists are crucial in medication management, ensuring optimal immunosuppression and postoperative care drug regimens. Effective interprofessional communication is paramount for seamless coordination among team members, facilitating the exchange of critical information for timely decision-making. Regular team meetings, case discussions, and shared electronic health records foster collaborative discussions. This collaborative approach enhances patient-centered care by addressing individual needs, improving patient outcomes through comprehensive care strategies, ensuring patient safety by minimizing medication errors and complications, and optimizing team performance through streamlined communication and coordination.
Nursing, Allied Health, and Interprofessional Team Interventions
The nursing, allied health staff, and the interprofessional team play a key role in managing cases with corneal transplantation. The nurses help in recruiting the patients to the outpatient department, help in scraping patients with microbial keratitis, explain the procedure to the patients, the importance of each keratoplasty, how to procure and use antimicrobial medication, steroids, and adjuvant drugs, help in counseling, postoperative management and follow up of these cases.[66]
Nursing, Allied Health, and Interprofessional Team Monitoring
The nursing, allied health staff, and the interprofessional team help monitor these patients with visual acuity assessment, intraocular pressure, regular follow-up, whether correct medications are being used, postoperative visual acuity, and regular monitoring and follow-up.[67]