Continuing Education Activity
The activity reviews the evaluation and management of carotid artery stenosis. It is important to differentiate between different etiologies of stroke, such as those caused by thrombosis, microemboli, hypoperfusion, or a cardioembolic source. Carotid stenosis is an important cause of stroke and hypoperfusion and is most commonly treated by carotid endarterectomy or medical management. Carotid stenting is also a therapeutic option in certain circumstances. Timing and management of interventions depend on the clinical presentation. Some of the variables to consider when determining treatments and timing of interventions are the presenting symptoms, ongoing or worsening neurologic symptoms, concomitant heart disease, and other comorbidities. Different guidelines also exist for those with carotid artery disease without neurologic symptoms. This activity reviews the pathophysiology, presentation, and diagnosis of carotid artery stenosis and discusses the role of the interprofessional team in its management.
Objectives:
Identify carotid artery stenosis risk factors and clinical indicators during patient assessments.
Implement evidence-based guidelines for the timely and individualized management of carotid artery stenosis, considering patient-specific factors and the severity of stenosis.
Apply the latest advancements in medical management, including lipid-lowering agents, blood pressure control, and diabetes management, to optimize care for patients with carotid artery stenosis.
Collaborate with multidisciplinary healthcare teams, including neurologists, vascular surgeons, and interventional radiologists, to ensure comprehensive and coordinated care for patients with carotid artery stenosis.
Introduction
Stroke is the third leading cause of death in developed countries and the leading cause of morbidity. The majority of strokes (85%) are ischemic in nature, and the most common source of occlusion is thought to be intracranial embolic disease from the ipsilateral carotid artery. Recurrent strokes are a significant concern as these often occur in the same vascular territory as the initial stroke and are associated with 65% mortality.[1]
Determining the difference between hemodynamic and embolic causes in the presence of internal carotid artery stenosis is important to determine the source of ischemia—usually presenting as classic stroke or transient ischemic attack (TIA). Symptoms may include contralateral motor or sensory deficits, as well as amaurosis fugax. Hemodynamic compromise may cause a presentation similar to a classic stroke or TIA but may also be less predictable and atypical. Patients have reported symptoms such as limb shaking, retinal claudication, headache from large pulsatile external carotid artery collaterals, syncope, and generalized fatigue. One-third of all strokes are related to cervical carotid disease. The mechanism of cervical carotid stroke is usually embolization from the carotid bifurcation plaque, but hemodynamic compromise from stenosis may also play a role. The risks of embolization and hemodynamic compromise increase the greater the degree of carotid artery stenosis.[2][3][4]
The benefit of carotid endarterectomy (CEA) depends on the degree of stenosis, with greater benefit associated with higher levels of stenosis. CEA is not a benign procedure, with an estimated 4% to 7% risk of stroke and death within the first 30 days of surgery. This is related to many factors but primarily to manipulation of intraluminal plaque. Periprocedural morbidity can also be difficult to quantify accurately because the natural history of stroke and TIA also results in a certain percentage of recurrent stroke and death.[1] Therefore, it is important to determine levels of stenosis at which the risk of CEA is outweighed by the benefit versus medical management alone. Optimal medical management should be applied to all patients and includes lipid-lowering agents, hypertension control, and diabetes management.
Timing of Carotid Endarterectomy
The timing of intervention for carotid stenosis is controversial because there is conflicting evidence about the risks and benefits of intervention performed at various time periods. The landmark trials North American Symptomatic Carotid Endarterectomy Trial (NASCET) and the European Carotid Surgery Trial (ECST), both published in 1991, showed that CEA for symptomatic carotid stenosis performed within 6 months of a symptomatic event reduces the rate of recurrent stroke, and most of the benefit was realized when CEA was performed within 2 weeks of the ischemic event. This led to recommendations that CEA be performed within 14 days of an ischemic event, with some guidelines using even stricter guidelines of CEA within 48 hours of the referring event.[5] Another study from the Swedish Vascular Registry (Swedvasc), published in 2012, suggested an increased risk of perioperative complications when CEA was performed within 48 hours of the ischemic event compared to CEA performed 3 to 7 days after the event (11.5% versus 3.6%). There was also no increased risk of embolic events in CEA performed at 8 to 14 days after the event.[5][6] More recent guidelines favor first classifying the index event as either a TIA or stroke—then considering CEA within 48 hours if the initial event is a TIA. If the initial event is a stroke, intervention between 3 to 7 days can be considered.[4] An exception to waiting 48 hours after the initial event would be patients who have repetitive or worsening TIAs, also called crescendo TIAs. These patients likely benefit from early intervention to avoid further neurologic compromise.[7] Multiple patient-specific factors play into the decision of optimal timing for a CEA.
Asymptomatic Carotid Artery Stenosis
Another area of contention is the presence of significant carotid artery stenosis in asymptomatic patients (patients without neurologic symptoms for the last six months.) Three randomized control trials studied whether CEA could reduce stroke risk in patients with asymptomatic carotid stenosis (usually >50% stenosis), comparing CEA with optimal medical management. These studies were the Veterans Affairs Cooperative Study Group (VA), the Asymptomatic Carotid Atherosclerosis Study (ACAS), and the Asymptomatic Carotid Surgery Trial-1 (ACST-1). In general, these studies found that stroke risk was significantly decreased in patients with asymptotic carotid stenosis, but there were limitations to these studies, including the following: there was a low incidence of ischemic stroke in both study groups; only surgeons with low complication rates were allowed to participate in two of these studies; and advances have been made in medical management of carotid stenosis since these studies were performed.[8][9]
Carotid Artery Stenosis and Coronary Artery Disease
Given that carotid stenosis is an atherosclerotic process, it is not surprising that a significant portion of patients with severe carotid stenosis have concurrent coronary artery disease (CAD). In patients who need coronary artery bypass graft surgery (CABG) and who are known to have severe (>70%) but asymptomatic carotid stenosis, the question arises regarding performing CEA before or at the same time as the CABG. A randomized control trial of 185 patients showed significant benefit for CEA either prior to or at the same time as CABG compared to patients who underwent CEA 1 to 3 months after CABG. The delayed CEA group had a significantly increased risk of perioperative or delayed stroke.[10]
Etiology
Carotid artery stenosis is a consequence of systemic atherosclerotic disease. Thus, any risk factor predisposing a patient to progressive atherosclerosis can potentially manifest as stenosis of the carotid artery with resultant ischemic stroke or TIA symptoms. Risks include smoking, hyperlipidemia, male gender, and increased age.[11][12][13][14]
In a minority of patients, especially young females with stenosis of the carotid artery, fibromuscular dysplasia (FMD) plays a much more significant role. FMD is a noninflammatory, nonatherosclerotic process affecting the carotid and renal arteries, although it can occur elsewhere in medium-sized vessels. FMD usually occurs in the mid and distal internal carotid artery, sometimes extending into the intracranial region. Aneurysms may also be a component of the disease process. Please see our companion article titled "Carotid Artery Fibromuscular Dysplasia."[15]
Epidemiology
Symptomatic internal carotid artery occlusion has an incidence of 6 per 100,000, though the rate of asymptomatic chronic occlusion is unknown and may be higher as imaging is not routinely done. Although patients who are Black and Hispanic have a higher risk of stroke than White patients, they have a lower incidence of severe stenosis (defined as >70%). This may explain lower rates of CEA in this population. Native Americans have a higher rate of severe stenosis than White patients. Males also have a higher rate of carotid artery disease than females.[16]
Pathophysiology
Total internal carotid artery occlusion results from thrombosis in the setting of chronic stenosis. Cardiogenic embolization to a normal carotid bifurcation or carotid dissection may also cause total occlusion of the internal carotid artery. Of note, if a cardioembolic source is present, symptoms may occur on the contralateral side of the stenosis, as emboli may be able to travel more easily through the less stenotic artery.
A previously asymptomatic chronic internal carotid artery occlusion may also become symptomatic if related to embolic or hemodynamic insults. Embolism may occur from the ipsilateral external carotid artery via collaterals to the cerebral circulation. It may also occur when there is occult patency of the occluded internal carotid artery, which then serves as the source of embolic material. Hemodynamic insufficiency may occur when any condition that interferes with cerebral perfusion, such as orthostasis, hypotension, volume depletion, or cardiac failure is superimposed on the carotid occlusion, especially when the contralateral carotid disease is significant.
Certain plaque characteristics are associated with an increased risk of embolization. Plaques that are echolucent or heterogenous on CT scan contain high lipid and blood components, respectively. These plaques are more likely to be ulcerated and pose a greater risk of stroke. One study showed that patients with symptomatic carotid stenosis had echolucent plaques 70% of the time, compared to asymptomatic patients who had only a 20% to 30% incidence of radiolucent plaques. Another study showed that patients with echolucent plaques had 2 to 4 times increased incidence of cerebral infarction compared to those who had echogenic plaques.[17]
History and Physical
Standard risk factors for coronary and systemic atherosclerosis also apply to carotid artery stenosis, such as increased age, male sex, family history, smoking, hypertension, hyperlipidemia, sedentary lifestyle, and high dietary fat. Usually, patients will present with recent neurologic symptoms—slurred speech, cranial nerve deficits, limb weakness, or visual disturbances. Amaurosis fugax is a common symptom of ipsilateral carotid stenosis causing emboli to the ophthalmic or retinal arteries.
Blood pressure in both arms and orthostatic blood pressure should be part of vital signs (especially in older patients). A detailed neurologic exam should be performed, including visual fields of both eyes. Carotid bruit is not a sensitive or specific finding for significant stenosis.[17] Cardiac exam may reveal an irregular heartbeat suggestive of atrial fibrillation or heart murmurs, which could indicate a cardioembolic or valvular source of stroke. A thorough skin and extremity exam should be performed to look for embolic skin lesions or changes of decreased vascularization, such as coolness in the extremities, loss of distal pulses, skin discoloration, and hair loss of the affected limb.
Evaluation
The physical exam should be coupled with diagnostic imaging (CT or MRI of the brain), followed by telemetry floor admission, as less than a third of ischemic strokes are due to carotid artery disease. The remaining are due to other causes, such as intracranial pathology or cardiac emboli. An EKG should also be performed, as well as a cardiac echo to look for embolic sources, including a patent foramen ovale.
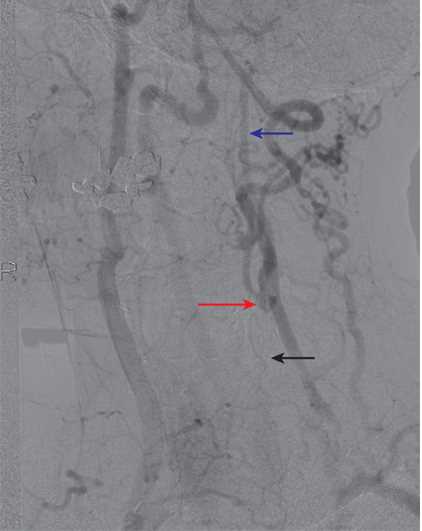
In diagnosing carotid artery stenosis, a common initial test is a carotid duplex, which is a noninvasive and inexpensive test. This examination typically shows a high-resistance signal in the carotid bulb and the proximal internal carotid artery. Distally, there are no Doppler signals audible in the carotid artery. As a confirmatory test, a contrast examination like a digital subtraction arteriography, magnetic resonance arteriography (MRA), or computed tomographic (CT) arteriography is required to confirm the diagnosis and plan a surgical approach if indicated. Gadolinium-enhanced MRA is better for differentiating high-grade stenosis than the time of flight angiography, which can overestimate the stenosis. MRI is considered the best modality to image not only the degree of stenosis, but also the characteristics of the plaque which leads to increased embolization. Plaque vulnerability is associated with the following: plaque ulceration and fissuring; necrosis of the lipid core; and intraplaque hemorrhage, thrombus, or inflammation.[18]
Treatment / Management
A large systematic review for the Cochrane database found that patients with severe stenosis of 70% to 99% definitively benefited from CEA, with a cumulative 5-year decrease in disabling/fatal stroke or perioperative death. Patients with moderate stenosis of 50% to 69% had a less pronounced, but still significant, decrease in the same parameter. In patients with moderate stenosis, this benefit was more pronounced in men than in women, who had higher surgical complication rates. Patients with 0% to 49% had more disabling/fatal strokes or perioperative death with CEA than medical management alone.[1] Patients who have near-occlusion (also described as having "trickle flow") are a separate category from patients with severe 70% to 99% stenosis because they have a lower risk of stroke. Earlier studies recommended only optimized medical treatment, but a recent large meta-analysis found that improvements in CEA and carotid stent procedures may warrant intervention in this situation.[19]
Chronic carotid artery occlusion accounts for about 6.5% of ischemic strokes.[20] There are no specific criteria for surveillance of patients with chronically occluded ICA, nor are there criteria to specify the degree of ECA stenosis by duplex ultrasound. A reasonable regimen is to follow the patient clinically every six months to a year, with or without a duplex study. The development of symptoms warrants imaging to see if cerebrovascular revascularization is indicated.
Carotid artery stenting (CAS) is preferred if symptomatic carotid occlusion (50-99%) is associated with multiple comorbidities, tracheostomy, prior neck radiation, or carotid dissection. Usually, there is an increased risk of stroke after CAS. Due to the advancements in stents and techniques, CAS is comparable to CEA in most instances. Studies suggest that CAS has a higher procedural rate of stroke (primarily non-disabling), but CEA has a significantly higher risk of procedural myocardial infarction.[8] A study on carotid revascularization for primary prevention of stroke (CREST-2) is ongoing and will shed more light on this important area.
In the setting of chronic total ICA occlusion, medical management is preferred over revascularization. However, several special clinical indications exist for possible procedural intervention, which is done on a case-by-case basis. Ipsilateral hemodynamic symptoms in the setting of ipsilateral ICA occlusion and contralateral ICA stenosis may benefit from contralateral ICA revascularization to ameliorate hemodynamic insufficiency. Ipsilateral embolic symptoms in the setting of ipsilateral ICA occlusion and ipsilateral external carotid occlusion (ECA) stenosis may be treated with ipsilateral ECA revascularization to eliminate the source of embolization, which occurs via enlarged ECA collaterals. Ligation of the ipsilateral ICA can also eliminate a source for embolization.[21][22][23]
Differential Diagnosis
The differential diagnosis of carotid artery stenosis may include the following: carotid artery dissection, fibromuscular dysplasia, valvular heart disease, arrhythmias (especially atrial fibrillation), mural thrombosis, Takayasu vasculitis, giant cell arteritis, and complicated migraine.
Prognosis
The risk of further stroke after successful CEA is associated with whether the initial ischemic event was a TIA or stroke. Patients who presented with TIA have an ipsilateral hemisphere stroke incidence of 1% to 2% per year, while those who presented with stroke have a corresponding risk of 2% to 3% per year.[17]
Complications
The main complication of carotid artery stenosis presenting with TIA, stroke, or other neurologic symptoms is recurrent stroke. The primary complications of CEA or CAS are also perioperative stroke, as well as surgical complications. In asymptomatic patients with carotid stenosis of 70% to 99%, the estimated rate of ipsilateral acute ischemic stroke was 4.7% over 5 years.[9]
Deterrence and Patient Education
After a suspected neurologic event, patients and family members should be educated regarding common symptoms of stroke and TIA, including dysarthria, numbness, weakness, tongue deviation, and confusion, as recurrent symptoms are common. Patients and family members should be advised to seek urgent medical attention for recurrent or new neurologic symptoms. Patients should be educated about medication adherence, especially antiplatelet agents, to prevent further strokes or TIA. They should also receive nutrition counseling to avoid foods high in cholesterol and fat.
Pearls and Other Issues
- Current guidelines support CEA for symptomatic carotid stenosis greater than 70%. Guidelines also recommend CEA for symptomatic stenosis of 50% to 69% with some leeway for variable clinical features.
- CEA should be performed within 14 days of the referring ischemic event. Urgent CEA (performed within 48 h) depends on whether the initial presentation was a stroke or TIA, with some evidence favoring urgent CEA in the setting of TIA.
- Unless contraindicated, crescendo TIA warrants urgent CEA or CAS.
- The guidelines surrounding asymptomatic stenosis are less well-defined, but they suggest CEA in patients with greater than 60% to 70% stenosis who are good surgical candidates.
- Of note, most studies of CEA versus optimal medical treatment selected surgeons with a known periprocedural complication rate of less than 6%.
- Carotid artery percutaneous stenting (CAS) is comparable to efficacy and complication rates of CEA and is preferred in patients with comorbidities preventing CEA.
- Ulcerated carotid plaques are highly associated with embolic stroke. Ulcerated plaques are radiolucent due to high lipid content, and ulcer size is also correlated with increased stroke risk.
- MRI is the best modality to image plaque characteristics indicating plaque vulnerability, such as ulceration, fissuring, a necrotic lipid core, or intraplaque hemorrhage.
- All patients, whether treated by CEA or medically, should receive aggressive lipid-lowering therapy, antiplatelet agents (dual anti-platelet agents in certain patients), and aggressive blood pressure management.
Enhancing Healthcare Team Outcomes
The optimal management of patients with carotid artery stenosis is with an interprofessional team, including primary care providers, internists, vascular surgeons, interventional radiologists, neurologists, and pharmacists. Post-stroke patients should be managed on a stroke floor with telemetry and dedicated nursing staff to conduct frequent neurologic checks. When a patient is discovered to have carotid stenosis, a referral should be made to a neurologist to determine the treatment plan. Evidence-based medical management should be performed in all at-risk patients and may include home nursing visits and patient education.