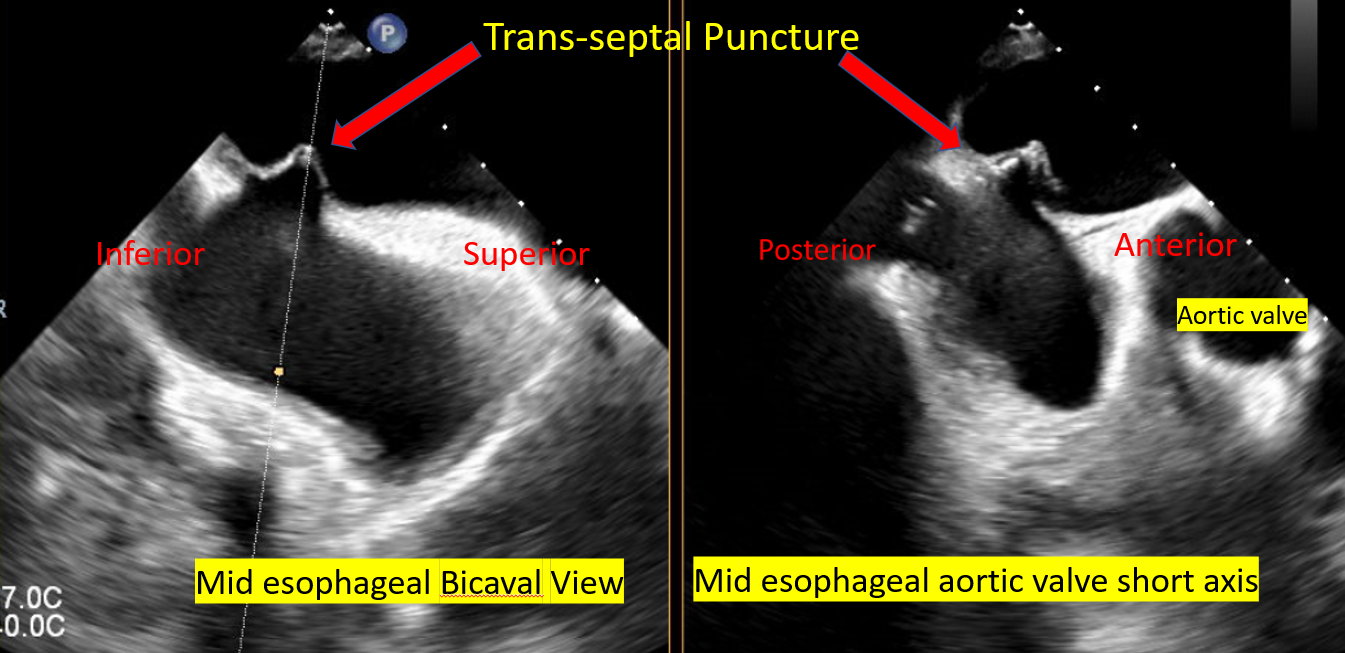
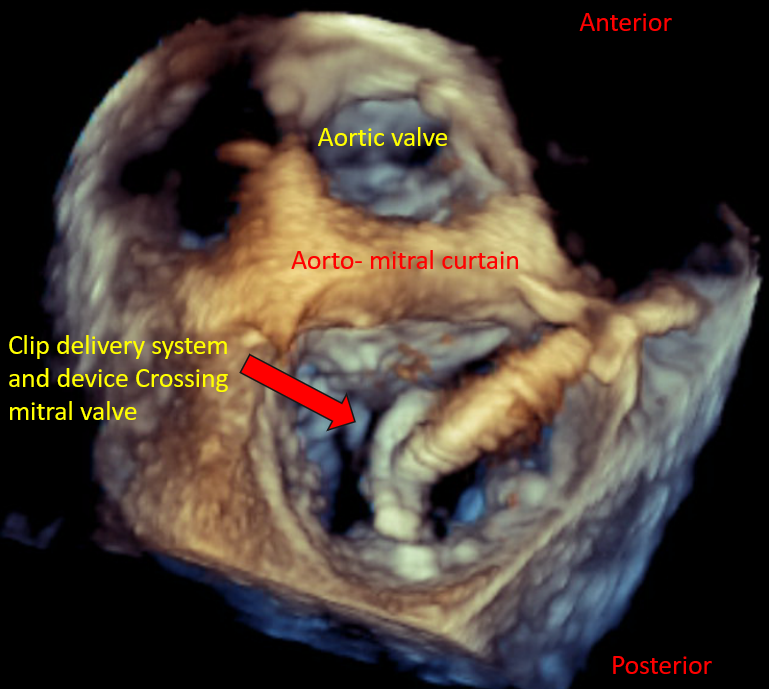
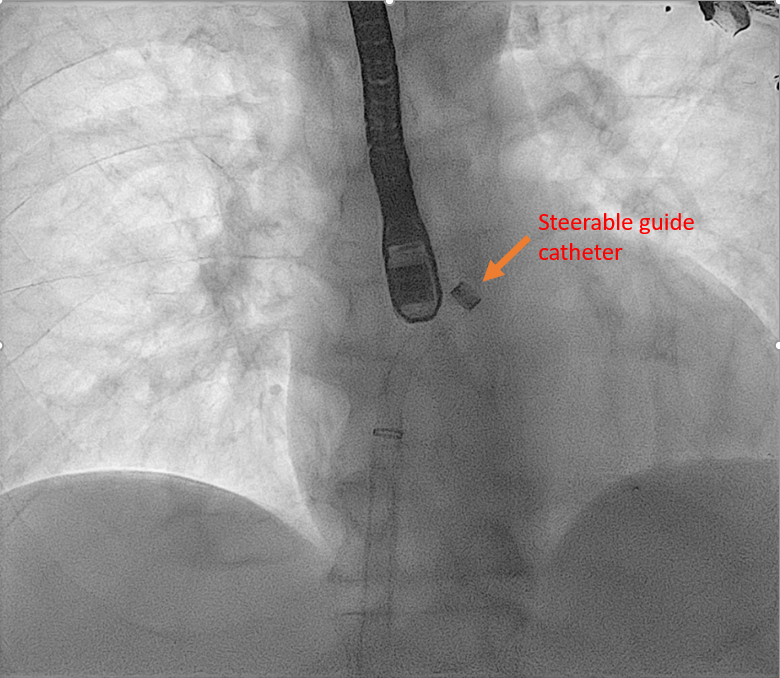
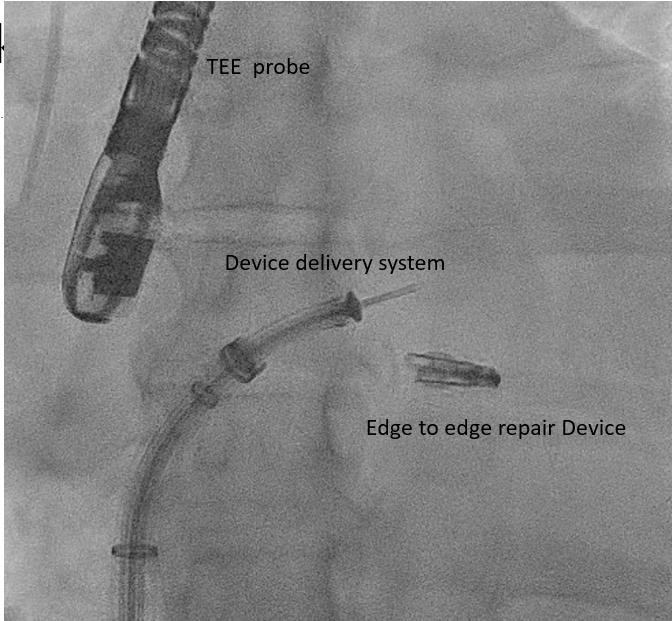
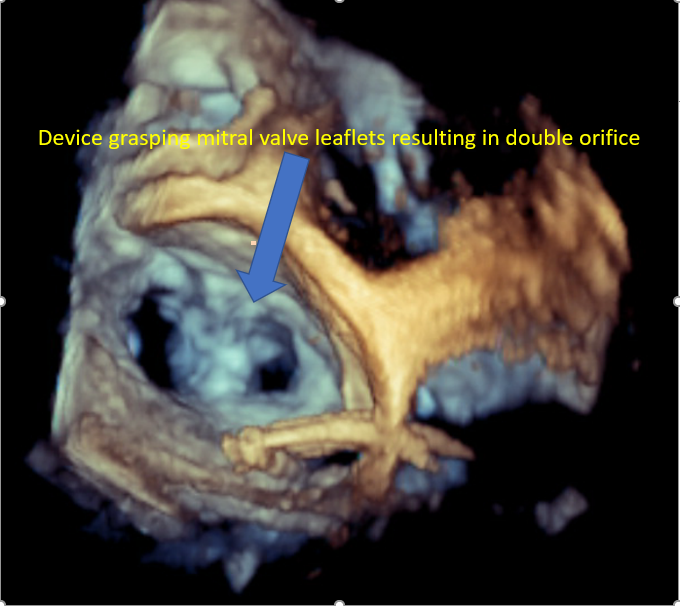
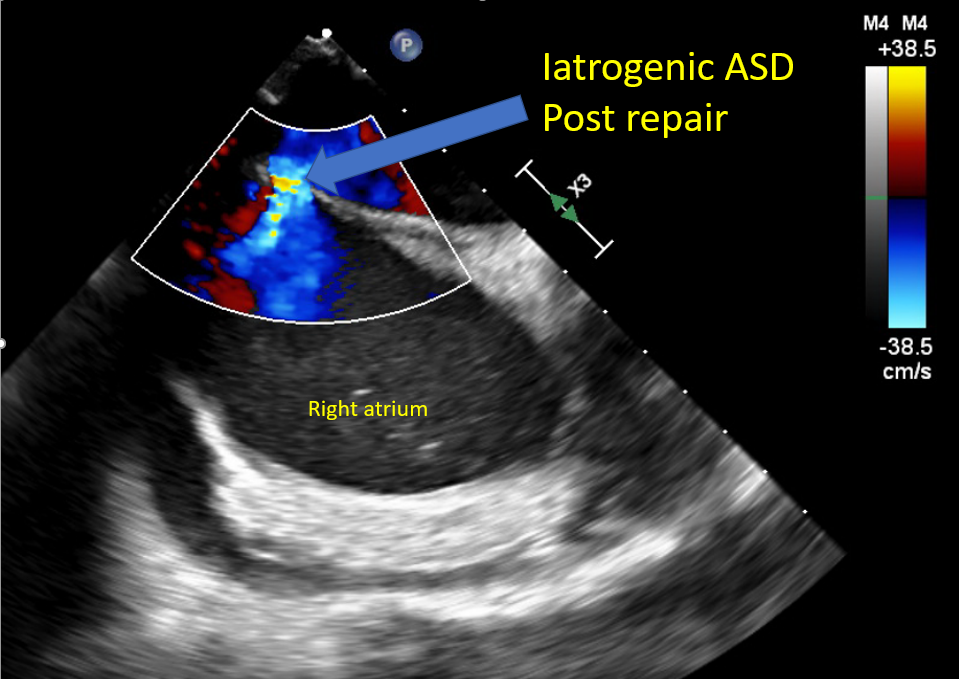
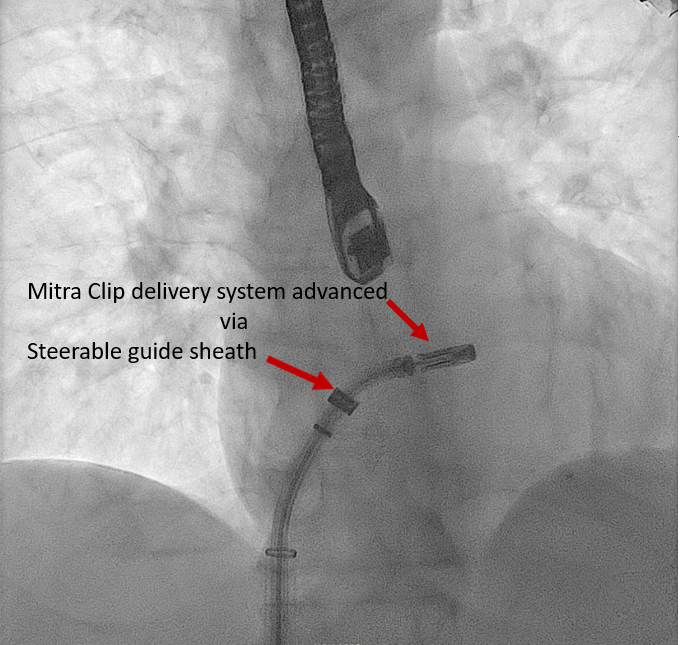
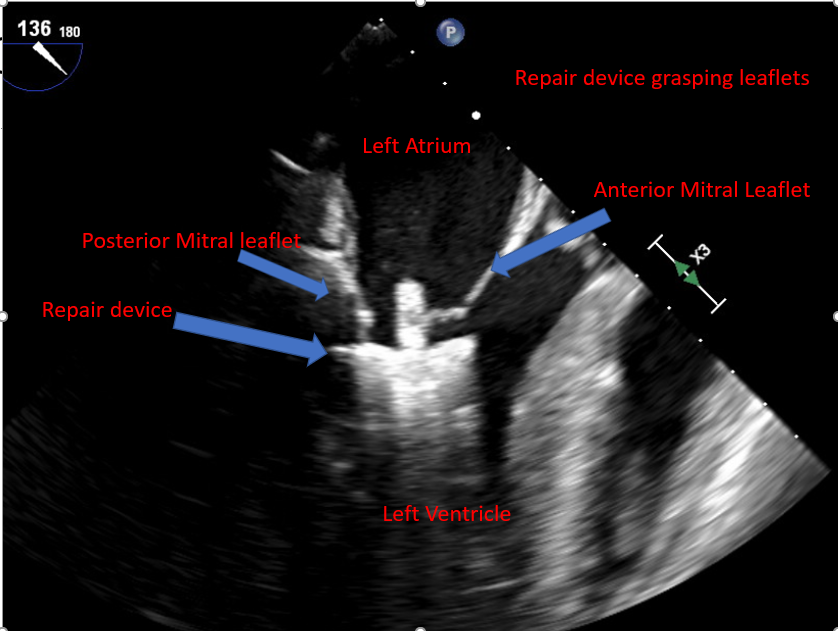
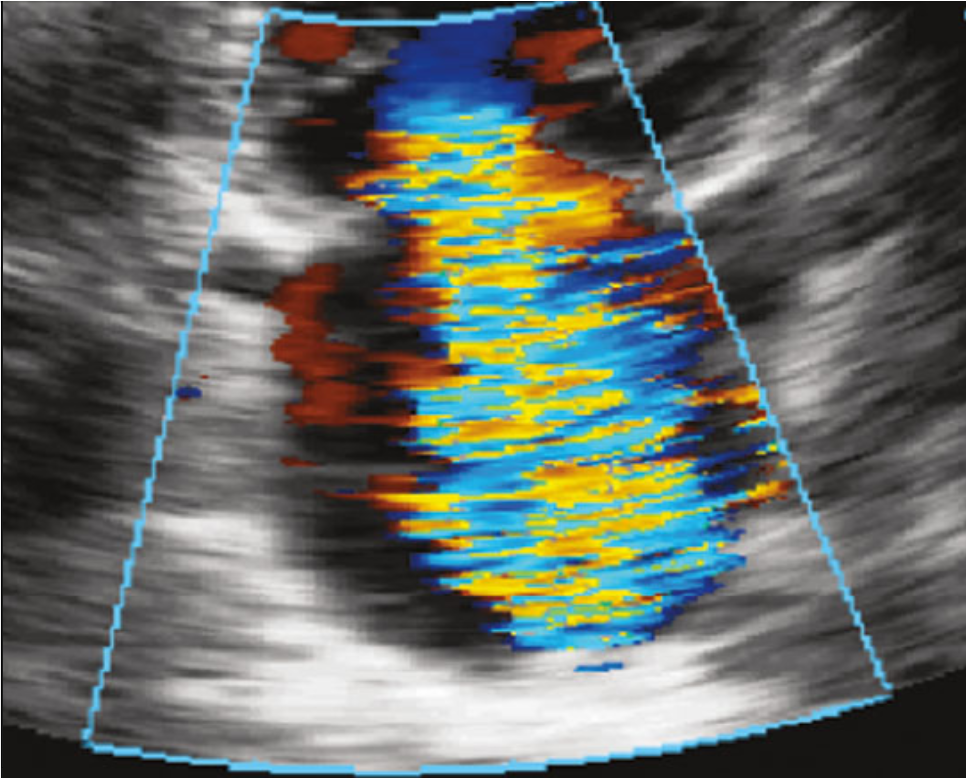
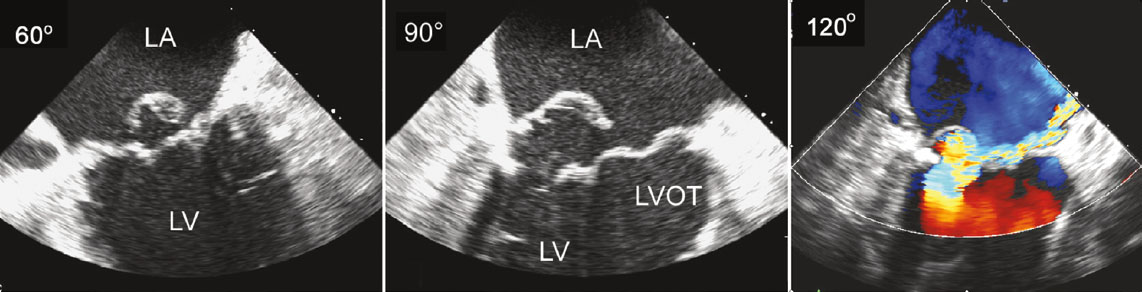
[1]
Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. European heart journal. 2003 Jul:24(13):1231-43
[PubMed PMID: 12831818]
Level 3 (low-level) evidence
[2]
Quader N, Rigolin VH. Two and three dimensional echocardiography for pre-operative assessment of mitral valve regurgitation. Cardiovascular ultrasound. 2014 Oct 25:12():42. doi: 10.1186/1476-7120-12-42. Epub 2014 Oct 25
[PubMed PMID: 25344779]
[3]
Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C, ACC/AHA Joint Committee Members, O'Gara PT, Beckman JA, Levine GN, Al-Khatib SM, Armbruster A, Birtcher KK, Ciggaroa J, Deswal A, Dixon DL, Fleisher LA, de Las Fuentes L, Gentile F, Goldberger ZD, Gorenek B, Haynes N, Hernandez AF, Hlatky MA, Joglar JA, Jones WS, Marine JE, Mark D, Palaniappan L, Piano MR, Spatz ES, Tamis-Holland J, Wijeysundera DN, Woo YJ. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. The Journal of thoracic and cardiovascular surgery. 2021 Aug:162(2):e183-e353. doi: 10.1016/j.jtcvs.2021.04.002. Epub 2021 May 8
[PubMed PMID: 33972115]
Level 1 (high-level) evidence
[4]
Guarracino F, Baldassarri R, Ferro B, Giannini C, Bertini P, Petronio AS, Di Bello V, Landoni G, Alfieri O. Transesophageal echocardiography during MitraClip® procedure. Anesthesia and analgesia. 2014 Jun:118(6):1188-96. doi: 10.1213/ANE.0000000000000215. Epub
[PubMed PMID: 24842173]
[5]
Vahanian A, Beyersdorf F, Praz F, Milojevic M, Baldus S, Bauersachs J, Capodanno D, Conradi L, De Bonis M, De Paulis R, Delgado V, Freemantle N, Gilard M, Haugaa KH, Jeppsson A, Jüni P, Pierard L, Prendergast BD, Sádaba JR, Tribouilloy C, Wojakowski W, ESC/EACTS Scientific Document Group. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Revista espanola de cardiologia (English ed.). 2022 Jun:75(6):524. doi: 10.1016/j.rec.2022.05.006. Epub
[PubMed PMID: 35636831]
[6]
Kang DH, Kim JH, Rim JH, Kim MJ, Yun SC, Song JM, Song H, Choi KJ, Song JK, Lee JW. Comparison of early surgery versus conventional treatment in asymptomatic severe mitral regurgitation. Circulation. 2009 Feb 17:119(6):797-804. doi: 10.1161/CIRCULATIONAHA.108.802314. Epub 2009 Feb 2
[PubMed PMID: 19188506]
[7]
Sidebotham DA, Allen SJ, Gerber IL, Fayers T. Intraoperative transesophageal echocardiography for surgical repair of mitral regurgitation. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2014 Apr:27(4):345-66. doi: 10.1016/j.echo.2014.01.005. Epub 2014 Feb 15
[PubMed PMID: 24534653]
[8]
Bonow RO, O'Gara PT, Adams DH, Badhwar V, Bavaria JE, Elmariah S, Hung JW, Lindenfeld J, Morris AA, Satpathy R, Whisenant B, Woo YJ. 2020 Focused Update of the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Solution Set Oversight Committee. Journal of the American College of Cardiology. 2020 May 5:75(17):2236-2270. doi: 10.1016/j.jacc.2020.02.005. Epub 2020 Feb 14
[PubMed PMID: 32068084]
Level 3 (low-level) evidence
[9]
Feldman T, Kar S, Rinaldi M, Fail P, Hermiller J, Smalling R, Whitlow PL, Gray W, Low R, Herrmann HC, Lim S, Foster E, Glower D, EVEREST Investigators. Percutaneous mitral repair with the MitraClip system: safety and midterm durability in the initial EVEREST (Endovascular Valve Edge-to-Edge REpair Study) cohort. Journal of the American College of Cardiology. 2009 Aug 18:54(8):686-94. doi: 10.1016/j.jacc.2009.03.077. Epub
[PubMed PMID: 19679246]
[10]
Feldman T, Kar S, Elmariah S, Smart SC, Trento A, Siegel RJ, Apruzzese P, Fail P, Rinaldi MJ, Smalling RW, Hermiller JB, Heimansohn D, Gray WA, Grayburn PA, Mack MJ, Lim DS, Ailawadi G, Herrmann HC, Acker MA, Silvestry FE, Foster E, Wang A, Glower DD, Mauri L, EVEREST II Investigators. Randomized Comparison of Percutaneous Repair and Surgery for Mitral Regurgitation: 5-Year Results of EVEREST II. Journal of the American College of Cardiology. 2015 Dec 29:66(25):2844-2854. doi: 10.1016/j.jacc.2015.10.018. Epub
[PubMed PMID: 26718672]
Level 1 (high-level) evidence
[11]
Feldman T, Foster E, Glower DD, Kar S, Rinaldi MJ, Fail PS, Smalling RW, Siegel R, Rose GA, Engeron E, Loghin C, Trento A, Skipper ER, Fudge T, Letsou GV, Massaro JM, Mauri L, EVEREST II Investigators. Percutaneous repair or surgery for mitral regurgitation. The New England journal of medicine. 2011 Apr 14:364(15):1395-406. doi: 10.1056/NEJMoa1009355. Epub 2011 Apr 4
[PubMed PMID: 21463154]
[12]
Alfieri O, Maisano F, De Bonis M, Stefano PL, Torracca L, Oppizzi M, La Canna G. The double-orifice technique in mitral valve repair: a simple solution for complex problems. The Journal of thoracic and cardiovascular surgery. 2001 Oct:122(4):674-81
[PubMed PMID: 11581597]
[13]
Mack MJ, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant BK, Grayburn PA, Rinaldi MJ, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Rogers JH, Marx SO, Cohen DJ, Weissman NJ, Stone GW, COAPT Investigators. 3-Year Outcomes of Transcatheter Mitral Valve Repair in Patients With Heart Failure. Journal of the American College of Cardiology. 2021 Mar 2:77(8):1029-1040. doi: 10.1016/j.jacc.2020.12.047. Epub
[PubMed PMID: 33632476]
[14]
Khatib D, Neuburger PJ, Pan S, Rong LQ. Transcatheter Mitral Valve Interventions for Mitral Regurgitation: A Review of Mitral Annuloplasty, Valve Replacement, and Chordal Repair Devices. Journal of cardiothoracic and vascular anesthesia. 2022 Oct:36(10):3887-3903. doi: 10.1053/j.jvca.2022.05.005. Epub 2022 May 8
[PubMed PMID: 35871885]
[15]
De Backer O, Piazza N, Banai S, Lutter G, Maisano F, Herrmann HC, Franzen OW, Søndergaard L. Percutaneous transcatheter mitral valve replacement: an overview of devices in preclinical and early clinical evaluation. Circulation. Cardiovascular interventions. 2014 Jun:7(3):400-9. doi: 10.1161/CIRCINTERVENTIONS.114.001607. Epub
[PubMed PMID: 24944303]
Level 3 (low-level) evidence
[16]
Ooms JF, Van Mieghem NM. Transcatheter Repair and Replacement Technologies for Mitral Regurgitation: a European Perspective. Current cardiology reports. 2021 Jul 16:23(9):125. doi: 10.1007/s11886-021-01556-6. Epub 2021 Jul 16
[PubMed PMID: 34269914]
Level 3 (low-level) evidence
[17]
Condado JA, Vélez-Gimón M. Catheter-based approach to mitral regurgitation. Journal of interventional cardiology. 2003 Dec:16(6):523-34
[PubMed PMID: 14632950]
[18]
Perloff JK, Roberts WC. The mitral apparatus. Functional anatomy of mitral regurgitation. Circulation. 1972 Aug:46(2):227-39
[PubMed PMID: 5046018]
[19]
Blanke P, Naoum C, Webb J, Dvir D, Hahn RT, Grayburn P, Moss RR, Reisman M, Piazza N, Leipsic J. Multimodality Imaging in the Context of Transcatheter Mitral Valve Replacement: Establishing Consensus Among Modalities and Disciplines. JACC. Cardiovascular imaging. 2015 Oct:8(10):1191-1208. doi: 10.1016/j.jcmg.2015.08.004. Epub
[PubMed PMID: 26481845]
Level 3 (low-level) evidence
[20]
El Sabbagh A, Reddy YNV, Nishimura RA. Mitral Valve Regurgitation in the Contemporary Era: Insights Into Diagnosis, Management, and Future Directions. JACC. Cardiovascular imaging. 2018 Apr:11(4):628-643. doi: 10.1016/j.jcmg.2018.01.009. Epub
[PubMed PMID: 29622181]
Level 3 (low-level) evidence
[21]
Dziadzko V, Clavel MA, Dziadzko M, Medina-Inojosa JR, Michelena H, Maalouf J, Nkomo V, Thapa P, Enriquez-Sarano M. Outcome and undertreatment of mitral regurgitation: a community cohort study. Lancet (London, England). 2018 Mar 10:391(10124):960-969. doi: 10.1016/S0140-6736(18)30473-2. Epub
[PubMed PMID: 29536860]
[22]
Shah PM, Raney AA. Echocardiography in mitral regurgitation with relevance to valve surgery. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2011 Oct:24(10):1086-91. doi: 10.1016/j.echo.2011.08.017. Epub
[PubMed PMID: 21933744]
[23]
Shiota T. Role of echocardiography for catheter-based management of valvular heart disease. Journal of cardiology. 2017 Jan:69(1):66-73. doi: 10.1016/j.jjcc.2016.09.015. Epub 2016 Nov 15
[PubMed PMID: 27863908]
[24]
Asch FM, Little SH, Mackensen GB, Grayburn PA, Sorajja P, Rinaldi MJ, Maisano F, Kar S. Incidence and standardised definitions of mitral valve leaflet adverse events after transcatheter mitral valve repair: the EXPAND study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2021 Dec 3:17(11):e932-e941. doi: 10.4244/EIJ-D-21-00012. Epub 2021 Dec 3
[PubMed PMID: 34031024]
[25]
Avierinos JF, Tribouilloy C, Grigioni F, Suri R, Barbieri A, Michelena HI, Ionico T, Rusinaru D, Ansaldi S, Habib G, Szymanski C, Giorgi R, Mahoney DW, Enriquez-Sarano M, Mitral regurgitation International DAtabase (MIDA) Investigators. Impact of ageing on presentation and outcome of mitral regurgitation due to flail leaflet: a multicentre international study. European heart journal. 2013 Sep:34(33):2600-9. doi: 10.1093/eurheartj/eht250. Epub 2013 Jul 12
[PubMed PMID: 23853072]
[26]
Thaden JJ, Malouf JF, Nkomo VT, Pislaru SV, Holmes DR Jr, Reeder GS, Rihal CS, Eleid MF. Mitral Valve Anatomic Predictors of Hemodynamic Success With Transcatheter Mitral Valve Repair. Journal of the American Heart Association. 2018 Jan 13:7(2):. doi: 10.1161/JAHA.117.007315. Epub 2018 Jan 13
[PubMed PMID: 29331957]
[27]
Estévez-Loureiro R, Franzen O, Winter R, Sondergaard L, Jacobsen P, Cheung G, Moat N, Ihlemann N, Ghione M, Price S, Duncan A, Streit Rosenberg T, Barker S, Di Mario C, Settergren M. Echocardiographic and clinical outcomes of central versus noncentral percutaneous edge-to-edge repair of degenerative mitral regurgitation. Journal of the American College of Cardiology. 2013 Dec 24:62(25):2370-2377. doi: 10.1016/j.jacc.2013.05.093. Epub 2013 Sep 4
[PubMed PMID: 24013059]
Level 2 (mid-level) evidence
[28]
Lapenna E, De Bonis M, Sorrentino F, La Canna G, Grimaldi A, Torracca L, Maisano F, Alfieri O. Commissural closure for the treatment of commissural mitral valve prolapse or flail. The Journal of heart valve disease. 2008 May:17(3):261-6
[PubMed PMID: 18592922]
[29]
Katz WE, Conrad Smith AJ, Crock FW, Cavalcante JL. Echocardiographic evaluation and guidance for MitraClip procedure. Cardiovascular diagnosis and therapy. 2017 Dec:7(6):616-632. doi: 10.21037/cdt.2017.07.04. Epub
[PubMed PMID: 29302467]
[30]
Khan F, Winkel M, Ong G, Brugger N, Pilgrim T, Windecker S, Praz F, Fam N. Percutaneous Mitral Edge-to-Edge Repair: State of the Art and a Glimpse to the Future. Frontiers in cardiovascular medicine. 2019:6():122. doi: 10.3389/fcvm.2019.00122. Epub 2019 Sep 18
[PubMed PMID: 31620446]
[31]
Gertz ZM, Herrmann HC, Lim DS, Kar S, Kapadia SR, Reed GW, Puri R, Krishnaswamy A, Gersh BJ, Weissman NJ, Asch FM, Grayburn PA, Kosmidou I, Redfors B, Zhang Z, Abraham WT, Lindenfeld J, Stone GW, Mack MJ. Implications of Atrial Fibrillation on the Mechanisms of Mitral Regurgitation and Response to MitraClip in the COAPT Trial. Circulation. Cardiovascular interventions. 2021 Apr:14(4):e010300. doi: 10.1161/CIRCINTERVENTIONS.120.010300. Epub 2021 Mar 15
[PubMed PMID: 33719505]
[32]
Abramowitz Y, Jilaihawi H, Chakravarty T, Mack MJ, Makkar RR. Mitral Annulus Calcification. Journal of the American College of Cardiology. 2015 Oct 27:66(17):1934-41. doi: 10.1016/j.jacc.2015.08.872. Epub
[PubMed PMID: 26493666]
[33]
Fernández-Peregrina E, Pascual I, Freixa X, Tirado-Conte G, Estévez-Loureiro R, Carrasco-Chinchilla F, Benito-González T, Asmarats L, Sanchís L, Jiménez-Quevedo P, Avanzas P, Caneiro-Queija B, Molina-Ramos AI, Fernández-Vázquez F, Li CH, Flores-Umanzor E, Sans-Roselló J, Nombela-Franco L, Arzamendi D. Transcatheter edge-to-edge mitral valve repair in patients with mitral annulus calcification. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2022 Mar 18:17(16):1300-1309. doi: 10.4244/EIJ-D-21-00205. Epub
[PubMed PMID: 34483091]
[34]
Flint N, Raschpichler M, Rader F, Shmueli H, Siegel RJ. Asymptomatic Degenerative Mitral Regurgitation: A Review. JAMA cardiology. 2020 Mar 1:5(3):346-355. doi: 10.1001/jamacardio.2019.5466. Epub
[PubMed PMID: 31995124]
[35]
Flameng W, Herijgers P, Bogaerts K. Recurrence of mitral valve regurgitation after mitral valve repair in degenerative valve disease. Circulation. 2003 Apr 1:107(12):1609-13
[PubMed PMID: 12668494]
[36]
Kim JH, Lee SH, Joo HC, Youn YN, Yoo KJ, Chang BC, Lee S. Effect of Recurrent Mitral Regurgitation After Mitral Valve Repair in Patients With Degenerative Mitral Regurgitation. Circulation journal : official journal of the Japanese Circulation Society. 2017 Dec 25:82(1):93-101. doi: 10.1253/circj.CJ-17-0380. Epub 2017 Jul 20
[PubMed PMID: 28724839]
[37]
Mehaffey HJ, Hawkins RB, Schubert S, Fonner C, Yarboro LT, Quader M, Speir A, Rich J, Kron IL, Ailawadi G. Contemporary outcomes in reoperative mitral valve surgery. Heart (British Cardiac Society). 2018 Apr:104(8):652-656. doi: 10.1136/heartjnl-2017-312047. Epub 2017 Oct 5
[PubMed PMID: 28982718]
[38]
Niikura H, Bae R, Gössl M, Lin D, Jay D, Sorajja P. Transcatheter therapy for residual mitral regurgitation after MitraClip therapy. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2019 Aug 9:15(6):e491-e499. doi: 10.4244/EIJ-D-18-01148. Epub 2019 Aug 9
[PubMed PMID: 31217145]
[39]
Braun D, Frerker C, Körber MI, Gaemperli O, Patzelt J, Schaefer U, Hammerstingl C, Boekstegers P, Ott I, Ince H, Thiele H, Hausleiter J. Percutaneous Edge-to-Edge Repair of Recurrent Severe Mitral Regurgitation After Surgical Mitral Valve Repair. Journal of the American College of Cardiology. 2017 Jul 25:70(4):504-505. doi: 10.1016/j.jacc.2017.05.045. Epub
[PubMed PMID: 28728696]
[40]
Grasso C, Ohno Y, Attizzani GF, Cannata S, Immè S, Barbanti M, Pistritto AM, Ministeri M, Caggegi A, Chiarandà M, Dipasqua F, Ronsivalle G, Mangiafico S, Scandura S, Capranzano P, Capodanno D, Tamburino C. Percutaneous mitral valve repair with the MitraClip system for severe mitral regurgitation in patients with surgical mitral valve repair failure. Journal of the American College of Cardiology. 2014 Mar 4:63(8):836-8. doi: 10.1016/j.jacc.2013.09.045. Epub 2013 Oct 23
[PubMed PMID: 24161329]
[41]
Kanda BS, Jay D, Farivar RS, Sorajja P. Leaflet-to-Annuloplasty Ring Clipping for Severe Mitral Regurgitation. JACC. Cardiovascular interventions. 2016 Apr 11:9(7):e63-4. doi: 10.1016/j.jcin.2015.12.272. Epub 2016 Mar 4
[PubMed PMID: 26952908]
[42]
Wu IY, Barajas MB, Hahn RT. The MitraClip Procedure-A Comprehensive Review for the Cardiac Anesthesiologist. Journal of cardiothoracic and vascular anesthesia. 2018 Dec:32(6):2746-2759. doi: 10.1053/j.jvca.2018.05.020. Epub 2018 Sep 27
[PubMed PMID: 30268642]
[43]
Hahn RT. Transcathether Valve Replacement and Valve Repair: Review of Procedures and Intraprocedural Echocardiographic Imaging. Circulation research. 2016 Jul 8:119(2):341-56. doi: 10.1161/CIRCRESAHA.116.307972. Epub
[PubMed PMID: 27390336]
[44]
Hahn RT, Chan V, Adams DH. Current Indications for Transcatheter Edge-to-Edge Repair in a Patient With Primary Mitral Regurgitation. Circulation. 2022 Oct 25:146(17):1263-1265. doi: 10.1161/CIRCULATIONAHA.122.061495. Epub 2022 Oct 24
[PubMed PMID: 36279415]
[45]
Lee G, Chikwe J, Milojevic M, Wijeysundera HC, Biondi-Zoccai G, Flather M, Gaudino MFL, Fremes SE, Tam DY. ESC/EACTS vs. ACC/AHA guidelines for the management of severe aortic stenosis. European heart journal. 2023 Mar 7:44(10):796-812. doi: 10.1093/eurheartj/ehac803. Epub
[PubMed PMID: 36632841]
[46]
Russell EA, Walsh WF, Costello B, McLellan AJA, Brown A, Reid CM, Tran L, Maguire GP. Medical Management of Rheumatic Heart Disease: A Systematic Review of the Evidence. Cardiology in review. 2018 Jul/Aug:26(4):187-195. doi: 10.1097/CRD.0000000000000185. Epub
[PubMed PMID: 29608495]
Level 1 (high-level) evidence
[47]
Van Praet KM, Stamm C, Sündermann SH, Meyer A, Unbehaun A, Montagner M, Nazari Shafti TZ, Jacobs S, Falk V, Kempfert J. Minimally Invasive Surgical Mitral Valve Repair: State of the Art Review. Interventional cardiology (London, England). 2018 Jan:13(1):14-19. doi: 10.15420/icr.2017:30:1. Epub
[PubMed PMID: 29593831]
[48]
Taramasso M, Gaemperli O, Maisano F. Treatment of degenerative mitral regurgitation in elderly patients. Nature reviews. Cardiology. 2015 Mar:12(3):177-83. doi: 10.1038/nrcardio.2014.210. Epub 2014 Dec 23
[PubMed PMID: 25533801]
[49]
Asch FM, Grayburn PA, Siegel RJ, Kar S, Lim DS, Zaroff JG, Mishell JM, Whisenant B, Mack MJ, Lindenfeld J, Abraham WT, Stone GW, Weissman NJ, COAPT Investigators. Echocardiographic Outcomes After Transcatheter Leaflet Approximation in Patients With Secondary Mitral Regurgitation: The COAPT Trial. Journal of the American College of Cardiology. 2019 Dec 17:74(24):2969-2979. doi: 10.1016/j.jacc.2019.09.017. Epub 2019 Sep 28
[PubMed PMID: 31574303]
[50]
Nishimura RA, Vahanian A, Eleid MF, Mack MJ. Mitral valve disease--current management and future challenges. Lancet (London, England). 2016 Mar 26:387(10025):1324-34. doi: 10.1016/S0140-6736(16)00558-4. Epub
[PubMed PMID: 27025438]
[51]
Chakravarty T, Makar M, Patel D, Oakley L, Yoon SH, Stegic J, Singh S, Skaf S, Nakamura M, Makkar RR. Transcatheter Edge-to-Edge Mitral Valve Repair With the MitraClip G4 System. JACC. Cardiovascular interventions. 2020 Oct 26:13(20):2402-2414. doi: 10.1016/j.jcin.2020.06.053. Epub 2020 Sep 30
[PubMed PMID: 33011141]
[52]
Praz F, Braun D, Unterhuber M, Spirito A, Orban M, Brugger N, Brinkmann I, Spring K, Moschovitis A, Nabauer M, Blazek S, Pilgrim T, Thiele H, Lurz P, Hausleiter J, Windecker S. Edge-to-Edge Mitral Valve Repair With Extended Clip Arms: Early Experience From a Multicenter Observational Study. JACC. Cardiovascular interventions. 2019 Jul 22:12(14):1356-1365. doi: 10.1016/j.jcin.2019.03.023. Epub 2019 May 22
[PubMed PMID: 31129091]
Level 2 (mid-level) evidence
[53]
Praz F, Spargias K, Chrissoheris M, Büllesfeld L, Nickenig G, Deuschl F, Schueler R, Fam NP, Moss R, Makar M, Boone R, Edwards J, Moschovitis A, Kar S, Webb J, Schäfer U, Feldman T, Windecker S. Compassionate use of the PASCAL transcatheter mitral valve repair system for patients with severe mitral regurgitation: a multicentre, prospective, observational, first-in-man study. Lancet (London, England). 2017 Aug 19:390(10096):773-780. doi: 10.1016/S0140-6736(17)31600-8. Epub
[PubMed PMID: 28831993]
[54]
Hausleiter J, Stocker TJ, Adamo M, Karam N, Swaans MJ, Praz F. Mitral valve transcatheter edge-to-edge repair. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2023 Jan 23:18(12):957-976. doi: 10.4244/EIJ-D-22-00725. Epub
[PubMed PMID: 36688459]
[55]
Huang EY, Chen C, Abdullah F, Aspelund G, Barnhart DC, Calkins CM, Cowles RA, Downard CD, Goldin AB, Lee SL, St Peter SD, Arca MJ, 2011 American Pediatric Surgical Association Outcomes and Clinical Trials Committee. Strategies for the prevention of central venous catheter infections: an American Pediatric Surgical Association Outcomes and Clinical Trials Committee systematic review. Journal of pediatric surgery. 2011 Oct:46(10):2000-11. doi: 10.1016/j.jpedsurg.2011.06.017. Epub
[PubMed PMID: 22008341]
Level 1 (high-level) evidence
[56]
Kassar M, Praz F, Hunziker L, Pilgrim T, Windecker S, Seiler C, Brugger N. Anatomical and Technical Predictors of Three-Dimensional Mitral Valve Area Reduction After Transcatheter Edge-To-Edge Repair. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2022 Jan:35(1):96-104. doi: 10.1016/j.echo.2021.08.021. Epub 2021 Oct 11
[PubMed PMID: 34506920]
[57]
Singh GD, Smith TW, Rogers JH. Multi-MitraClip therapy for severe degenerative mitral regurgitation: "anchor" technique for extremely flail segments. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2015 Aug:86(2):339-46. doi: 10.1002/ccd.25811. Epub 2015 Feb 12
[PubMed PMID: 25559345]
[58]
Oguz D, Padang R, Rashedi N, Pislaru SV, Nkomo VT, Mankad SV, Malouf JF, Guerrero M, Reeder GS, Eleid MF, Rihal CS, Thaden JJ. Risk for Increased Mean Diastolic Gradient after Transcatheter Edge-to-Edge Mitral Valve Repair: A Quantitative Three-Dimensional Transesophageal Echocardiographic Analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2021 Jun:34(6):595-603.e2. doi: 10.1016/j.echo.2021.01.018. Epub 2021 Jan 29
[PubMed PMID: 33524491]
[59]
Naqvi TZ. Echocardiography in percutaneous valve therapy. JACC. Cardiovascular imaging. 2009 Oct:2(10):1226-37. doi: 10.1016/j.jcmg.2009.08.004. Epub
[PubMed PMID: 19833314]
[60]
Hahn RT, Saric M, Faletra FF, Garg R, Gillam LD, Horton K, Khalique OK, Little SH, Mackensen GB, Oh J, Quader N, Safi L, Scalia GM, Lang RM. Recommended Standards for the Performance of Transesophageal Echocardiographic Screening for Structural Heart Intervention: From the American Society of Echocardiography. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2022 Jan:35(1):1-76. doi: 10.1016/j.echo.2021.07.006. Epub 2021 Jul 17
[PubMed PMID: 34280494]
[61]
Lancellotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL, Scientific Document Committee of the European Association of Cardiovascular Imaging. Recommendations for the echocardiographic assessment of native valvular regurgitation: an executive summary from the European Association of Cardiovascular Imaging. European heart journal. Cardiovascular Imaging. 2013 Jul:14(7):611-44. doi: 10.1093/ehjci/jet105. Epub 2013 Jun 3
[PubMed PMID: 23733442]
[62]
Alkhouli M, Rihal CS, Holmes DR Jr. Transseptal Techniques for Emerging Structural Heart Interventions. JACC. Cardiovascular interventions. 2016 Dec 26:9(24):2465-2480. doi: 10.1016/j.jcin.2016.10.035. Epub
[PubMed PMID: 28007198]
[63]
Sherif MA, Paranskaya L, Yuecel S, Kische S, Thiele O, D'Ancona G, Neuhausen-Abramkina A, Ortak J, Ince H, Öner A. MitraClip step by step; how to simplify the procedure. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2017 Feb:25(2):125-130. doi: 10.1007/s12471-016-0930-7. Epub
[PubMed PMID: 27933588]
[64]
Swaans MJ, Van den Branden BJ, Van der Heyden JA, Post MC, Rensing BJ, Eefting FD, Plokker HW, Jaarsma W. Three-dimensional transoesophageal echocardiography in a patient undergoing percutaneous mitral valve repair using the edge-to-edge clip technique. European journal of echocardiography : the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009 Dec:10(8):982-3. doi: 10.1093/ejechocard/jep101. Epub 2009 Aug 4
[PubMed PMID: 19654135]
[65]
Maslow A, Mahmood F, Poppas A, Singh A. Three-dimensional echocardiographic assessment of the repaired mitral valve. Journal of cardiothoracic and vascular anesthesia. 2014 Feb:28(1):11-17. doi: 10.1053/j.jvca.2013.05.007. Epub 2013 Sep 25
[PubMed PMID: 24075641]
[66]
Saitoh T, Izumo M, Furugen A, Tanaka J, Miyata-Fukuoka Y, Gurudevan SV, Tolstrup K, Siegel RJ, Kar S, Shiota T. Echocardiographic evaluation of iatrogenic atrial septal defect after catheter-based mitral valve clip insertion. The American journal of cardiology. 2012 Jun 15:109(12):1787-91. doi: 10.1016/j.amjcard.2012.02.023. Epub 2012 Apr 3
[PubMed PMID: 22475361]
[67]
Writing Committee Members, Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, Jneid H, Krieger EV, Mack M, McLeod C, O'Gara PT, Rigolin VH, Sundt TM 3rd, Thompson A, Toly C. 2020 ACC/AHA Guideline for the Management of Patients With Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Journal of the American College of Cardiology. 2021 Feb 2:77(4):450-500. doi: 10.1016/j.jacc.2020.11.035. Epub 2020 Dec 17
[PubMed PMID: 33342587]
[68]
Nickenig G, Estevez-Loureiro R, Franzen O, Tamburino C, Vanderheyden M, Lüscher TF, Moat N, Price S, Dall'Ara G, Winter R, Corti R, Grasso C, Snow TM, Jeger R, Blankenberg S, Settergren M, Tiroch K, Balzer J, Petronio AS, Büttner HJ, Ettori F, Sievert H, Fiorino MG, Claeys M, Ussia GP, Baumgartner H, Scandura S, Alamgir F, Keshavarzi F, Colombo A, Maisano F, Ebelt H, Aruta P, Lubos E, Plicht B, Schueler R, Pighi M, Di Mario C, Transcatheter Valve Treatment Sentinel Registry Investigators of the EURObservational Research Programme of the European Society of Cardiology. Percutaneous mitral valve edge-to-edge repair: in-hospital results and 1-year follow-up of 628 patients of the 2011-2012 Pilot European Sentinel Registry. Journal of the American College of Cardiology. 2014 Sep 2:64(9):875-84. doi: 10.1016/j.jacc.2014.06.1166. Epub
[PubMed PMID: 25169171]
Level 3 (low-level) evidence
[69]
Schnitzler K, Hell M, Geyer M, Kreidel F, Münzel T, von Bardeleben RS. Complications Following MitraClip Implantation. Current cardiology reports. 2021 Aug 13:23(9):131. doi: 10.1007/s11886-021-01553-9. Epub 2021 Aug 13
[PubMed PMID: 34387748]
[70]
Sticchi A, Bartkowiak J, Brugger N, Weiss S, Windecker S, Praz F. Retrograde Retrieval of a Novel Large Mitral Clip After Embolization Into the Left Ventricle. JACC. Case reports. 2021 Oct 20:3(14):1561-1568. doi: 10.1016/j.jaccas.2021.08.024. Epub 2021 Oct 20
[PubMed PMID: 34729501]
Level 3 (low-level) evidence
[71]
Hamm K, Barth S, Diegeler A, Kerber S. Stroke and thrombus formation appending to the MitraClip: what is the appropriate anticoagulation regimen? The Journal of heart valve disease. 2013 Sep:22(5):713-5
[PubMed PMID: 24383386]
[72]
Nakajima Y, Kar S. First experience of the usage of a GORE CARDIOFORM Septal Occluder device for treatment of a significant residual commissural mitral regurgitation jet following a MitraClip procedure. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2018 Sep 1:92(3):607-610. doi: 10.1002/ccd.27438. Epub 2017 Dec 8
[PubMed PMID: 29219253]
[73]
Mangieri A, Melillo F, Montalto C, Denti P, Praz F, Sala A, Winkel MG, Taramasso M, Tagliari AP, Fam NP, Rubbio AP, De Marco F, Bedogni F, Toggweiler S, Schofer J, Brinkmann C, Sievert H, Van Mieghem NM, Ooms JF, Paradis JM, Rodés-Cabau J, Brochet E, Himbert D, Perl L, Kornowski R, Ielasi A, Regazzoli D, Baldetti L, Masiero G, Tarantini G, Latib A, Laricchia A, Gattas A, Tchetchè D, Dumonteil N, Francesco G, Agricola E, Montorfano M, Lurz P, Crimi G, Maisano F, Colombo A. Management and Outcome of Failed Percutaneous Edge-to-Edge Mitral Valve Plasty: Insight From an International Registry. JACC. Cardiovascular interventions. 2022 Feb 28:15(4):411-422. doi: 10.1016/j.jcin.2021.11.040. Epub
[PubMed PMID: 35210047]
[74]
Alessandrini H, Dreher A, Harr C, Wohlmuth P, Meincke F, Hakmi S, Ubben T, Kuck KH, Hassan K, Willems S, Schmoeckel M, Geidel S. Clinical impact of intervention strategies after failed transcatheter mitral valve repair. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2021 Apr 20:16(17):1447-1454. doi: 10.4244/EIJ-D-20-01008. Epub
[PubMed PMID: 33074154]
[75]
Lavall D, Reil JC, Segura Schmitz L, Mehrer M, Schirmer SH, Böhm M, Laufs U. Early Hemodynamic Improvement after Percutaneous Mitral Valve Repair Evaluated by Noninvasive Pressure-Volume Analysis. Journal of the American Society of Echocardiography : official publication of the American Society of Echocardiography. 2016 Sep:29(9):888-98. doi: 10.1016/j.echo.2016.05.012. Epub 2016 Jun 29
[PubMed PMID: 27372560]