Continuing Education Activity
Malaria is an infection caused by Plasmodium species endemic to most parts of Africa, South America, East Asia, and parts of Europe and the Middle East. At least 10 to 30 thousand of the 125 million travelers to these areas are infected each year. All visitors to endemic areas should receive counseling on malaria risk, mosquito bite avoidance, and tailored chemoprophylaxis based on their medical histories and travel plans. This activity reviews the evaluation and management of chemoprophylaxis of malaria and highlights the role of the healthcare team in improving care for patients with potential exposure to this condition.
Objectives:
- Describe common options for malaria chemoprophylaxis.
- Identify common adverse events associated with antimalarial drugs.
- Outline the critical differences in malaria prophylaxis amongst special populations.
- Explain the importance of improving care coordination amongst the interprofessional team to enhance the delivery of care for patients with potential exposure to malaria.
Introduction
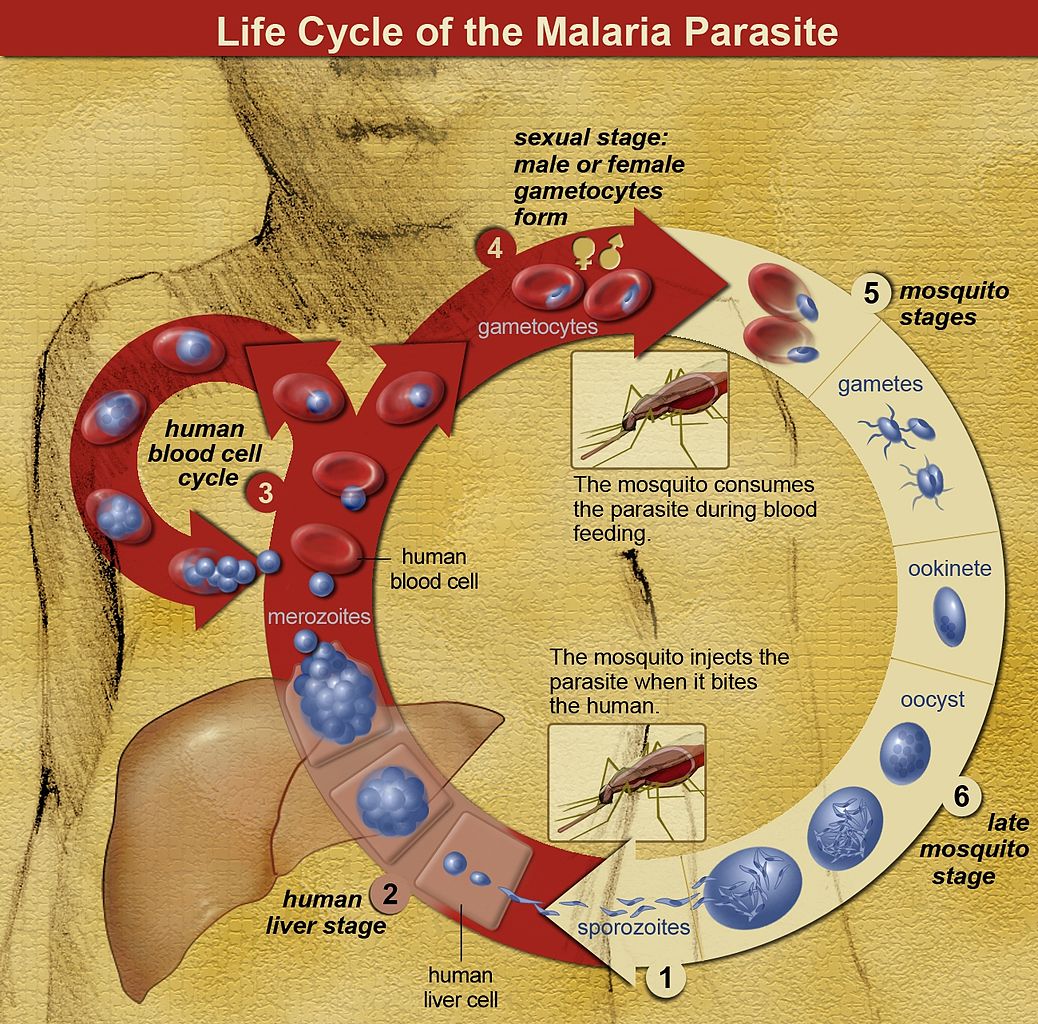
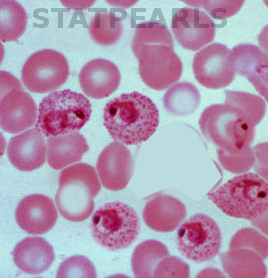
Malaria is an infection caused by one of five species of Plasmodium and is one of the leading causes of fever in the returning traveler. The Plasmodium species that cause disease in humans are Plasmodium falciparum, Plasmodium vivax, Plasmodium ovale, Plasmodium malariae, and Plasmodium knowlesi. The infected Anopheles mosquito, most active in the morning and evening, transmits the protozoa to humans. After transmission by an infected mosquito, the Plasmodium sporozoite infects and multiplies within liver cells. These schizonts then get released from liver cells, where they infect red blood cells and again multiply. Clinical disease results from the release of Plasmodium merozoites from the red blood cell. Also, Plasmodium vivax and Plasmodium ovale can remain dormant in the liver (hypnozoites) and can cause disease weeks to years later.[1]
Malaria is endemic to most parts of Africa, South America, East Asia, and parts of Europe and the Middle East. Approximately 125 million visitors travel to these areas, and estimates of malaria infections in travelers range between ten to thirty thousand though the actual number of cases is likely far higher. All travelers to endemic areas should, therefore, receive counseling on areas with a malaria risk, mosquito bite avoidance, and chemoprophylaxis.
Function
General Considerations
A pre-travel evaluation is necessary for the traveler to a malaria-endemic area. The patient’s past medical history, including psychiatric history, current medications, pregnancy status, and allergies, should be obtained. When considering chemoprophylaxis, providers should consider the traveler’s comorbidities, medication allergies, itinerary, activities, length of travel in endemic areas, and medication cost. Providers should research Plasmodium species endemic to the area of travel as well as resistance patterns, seasonality, and intensity of transmission in the area to be visited. Travelers should receive counseling on the prevention of mosquito bites, including wearing clothing that reduces exposed skin, using DEET on any exposed skin, staying in well-screened rooms, sleeping under permethrin-treated bed nets, and avoiding the outdoors at both dawn and dusk. When using sunscreen and mosquito repellant simultaneously, apply insect repellant after sunscreen. Women should be screened and asked about anticipated pregnancy, as this will affect prophylactic choices.
Multiple resources are available for researching malaria-endemic areas and resistance patterns as part of the pre-travel assessment. The Centers for Disease Control and Prevention and the World Health Organization offer free resources for providers and travelers with detailed information. Subscription sites also provide detailed information that clinicians and travelers may reference.
Chemoprophylaxis Choices
Malarial chemoprophylaxis functions by targeting the liver schizont, blood schizont, or hypnozoite stages of the plasmodium life cycle. The three most commonly prescribed medications for chemoprophylaxis are atovaquone-proguanil, doxycycline, and mefloquine. Studies examining the efficacy of the medications have found them all to be equally effective in the prevention of malaria in short-term travelers, but atovaquone-proguanil and doxycycline appear to have the fewest side effects. For long term travelers, studies suggest that adherence is lower in young travelers and those on mefloquine.
Atovaquone-proguanil: Atovaquone-proguanil targets both liver and blood schizonts and is effective against chloroquine-resistant P. falciparum. Dosing begins 1 to 2 days before the traveler enters the malarial zone, and one tablet is taken at the same time each day until seven days after exiting the malarial zone. The medication is relatively expensive compared to other regimens but has a longer half-life and is, therefore, more forgiving if a traveler misses a dose. The drug is well-tolerated; side effects include gastrointestinal upset, headaches, and transaminitis. Atovaquone-proguanil is not indicated for pregnant women, children under 5kg, or patients with creatinine clearance less than 30mL/min. There is no evidence regarding the clinical impact of co-administering warfarin and atovaquone-proguanil, but monitoring of the international normalized ratio may be required.[2]
Doxycycline: Doxycycline targets blood schizonts and is effective against chloroquine-resistant P. falciparum. The medication is started 1 to 2 days before travel and continued daily until four weeks after return. Side effects include GI upset, pill esophagitis, and photosensitivity. Doxycycline is typically the most inexpensive of the medications. The short half-life of the drug, however, results in a lack of protection if a traveler misses even a single dose. When taken as indicated, doxycycline has been shown to have an efficacy rate of 92 to 96% for P. falciparum and 98% for P. vivax.
Mefloquine: Mefloquine targets blood schizonts and is effective against chloroquine-resistant P. falciparum, though mefloquine resistance has emerged in parts of Southeast Asia. Travelers begin the medication at least two weeks before travel and take it weekly until four weeks after return. Neuropsychiatric adverse effects include seizures and psychosis. Providers should avoid the drug in patients with these histories as well as recent or active depression, schizophrenia, or generalized anxiety disorder. Other adverse effects include vivid dreams, gastrointestinal upset, and headaches. Many prescribers will start the medication 1 to 2 months before travel to assess for these adverse effects. An FDA black-box warning exists with rare reports of persistent dizziness following mefloquine use.
Chloroquine and hydroxychloroquine: Chloroquine targets blood schizonts. There is considerable resistance to these medications, and they should only merit consideration for travelers to areas with chloroquine-sensitive P. falciparum (the Caribbean, Central America west of the Panama Canal, and some parts of the Middle East). Travelers start the medication one week before travel and take it once every seven days until four weeks after return. The medication is usually well-tolerated, but side effects include gastrointestinal upset, headache, dizziness, and flaring of pre-existing psoriasis. The retinopathy associated with high doses in the treatment of rheumatoid arthritis is unlikely when dosing weekly for malarial prophylaxis.
Primaquine: Primaquine targets liver hypnozoites and can be used as prophylaxis in areas with only P. vivax. Travelers begin the medication two days before travel and take it daily until seven days after return. Primaquine is more common as presumptive antirelapse therapy (PART), where it is taken daily upon returning from a P. vivax or P. ovale endemic area in the final 14 days of post-travel treatment. The exception is when atovaquone-proguanil was used for primary prevention, in which case primaquine gets administered along with the final seven days of the primary prevention drug and for an additional seven days. Providers must test travelers for glucose-6-phosphate dehydrogenase (G6PD) deficiency before prescribing the medication as it can cause fatal hemolytic anemia. Side effects consist mostly of gastrointestinal upset. Prophylaxis with primaquine is currently an off-label recommendation from the CDC.[3][4]
Tafenoquine: Tafenoquine is active against hypnotize and erythrocytic forms of Plasmodium species. It also has activity against pre-erythrocytic forms, which prevents relapses of P. vivax. It is approved for prophylaxis for those over 16 years old. Dosing is daily for three days before travel to an endemic area, then weekly until seven days after return from travel. This drug also may cause life-threatening hemolytic anemia in patients with G6PD deficiency, and testing is essential before initiating the regimen. There are reports of psychiatric side effects, and use should discontinue in cases of psychotic symptoms. Other side effects include GI upset, headache, asymptomatic elevations in methemoglobin levels and epithelial keratopathy. Because the medication has a half-life of 17 days, delayed presentations of adverse reactions may occur.[3]
Fewer than 50% of travelers who contracted malaria sought pre-travel consultation. Those at particular risk for malaria include those taking last-minute vacations and those visiting family and friends abroad, often because they do not believe they are at risk of contracting malaria or do not see the disease as particularly dangerous. These risks may be due to the erroneous belief that they carry lifelong immunity to malaria even after emigrating from the endemic area.[5][6]
Vaccines
Addressing challenges of longterm immunity and durable protection are all currently underway.[7] Though researchers have attempted to develop a vaccine for decades, it has proven difficult due to the complicated lifecycle and antigenic changes of the protozoa. A pharmaceutical manufacturer, in collaboration with the Walter Reed Army Institute of Research, developed the RTS, S/AS01 vaccine, an injectable vaccine that helps mitigate the severity of P. falciparum infection. The vaccine prevents infection, maturation, and multiplication within the liver. A Phase 3 trial examining the vaccine efficacy in African children aged 5 to 17 months found a 39% reduction in malaria infection and a 31% reduction in severe malaria over 48 months.[8] This efficacy, however, appeared to wane over time, particularly in high endemic areas.[9] The WHO, via the Malaria Vaccine Implementation Program, is currently administering the vaccine in Ghana, Kenya, and Malawi. There are presently no vaccines available for travelers.
Issues of Concern
Special Populations
Pregnant travelers should generally be counseled to avoid travel to malaria-endemic areas until after delivery as malaria increases the risk for prematurity, spontaneous abortion, and stillbirth. Malaria may also be more severe in pregnant patients. In situations where deferring travel is not possible, mefloquine, chloroquine, and hydroxychloroquine are safe for pregnancy when accounting for the usual contraindications and resistance patterns. Doxycycline is contraindicated in pregnant women due to concerns for fetal dental discoloration and bone growth inhibition. Atovaquone-proguanil has not been well-studied in pregnant women and is generally not prescribed in this population. Primaquine should also not be used in pregnant travelers due to concerns about fetal G6PD deficiency.
Breastfeeding: There is not enough antimalarial drug excreted in breastmilk to provide chemoprophylaxis to infants. As mefloquine, chloroquine, and hydroxychloroquine are safe for infants; they are also reasonable during breastfeeding. Little data exists for doxycycline, but it is generally not recommended for use in the breastfeeding mother. Atovaquone-proguanil also has little safety data and is not recommended for prophylaxis in those breastfeeding infants under 5 kg. Primaquine also has no available data; however, both the mother and the infant require G6PD deficiency testing before prophylaxis with primaquine. Tafenoquine is also not recommended due to a lack of data.
Children Atovaquone-proguanil is an option for children over 5 kg. Mefloquine, chloroquine, and primaquine can be prescribed to children, taking into account the usual contraindications and resistance patterns. Children under the age of 8 should not receive doxycycline due to concerns of dental discoloration. Pediatric weight-based doses should not exceed adult doses and may be tolerated best when mixed with something sweet to overcome the bitter taste. The medication should also be taken on a full stomach to reduce the risk of vomiting or other GI upset. Overdose of antimalarials, especially chloroquine, can be fatal to children - childproof bottles and regular safety measures are essential.
Malaria prevention in older travelers: Elderly travelers are more likely to suffer morbidity and mortality related to malaria than their younger counterparts. In a systematic review, travelers over 60 years old were less likely to adhere to mosquito avoidance measures, though chemoprophylaxis adherence appeared better in older patients. Prescribers should consider any creatinine clearance inhibition when choosing chemoprophylaxis regimens.[10]
Clinical Significance
In laboratory testing, atovaquone-proguanil and mefloquine have 98% efficacy against malaria. The patient’s compliance and tolerance dictate the efficacy of the drug choice. New medications that can reduce side effects such as GI upset, psychiatric events, and seizures will lead to better-tolerated regimens with clinical efficacy similar to that seen in the lab.[3]
Other Issues
Obtaining medication abroad: While many drugs used in malaria prophylaxis are obtainable abroad, travelers should understand that the quality of these products is uncertain. Often they are also found in combination products with medications that may not be advisable. Finally, patients should typically begin their chemoprophylaxis regimen before travel. Patients should be discouraged from obtaining malaria prophylaxis while traveling abroad.
Changing prophylaxis regimens: If a patient cannot tolerate the side effects of an antimalarial drug, it is essential to consider the different parts of the lifecycle that each drug acts upon to ensure the best coverage. References such as those in the CDC Yellow Book can suggest the best regimen, taking into account the current medication, when the patient will depart a malarial zone, and other important factors.
Enhancing Healthcare Team Outcomes
Healthcare workers, including the primary care clinician and advanced nurse practitioner, should be familiar with malaria prophylaxis. The interprofessional healthcare team should take care to provide access and culturally competent guidance to those visiting friends and relatives in endemic areas. Providers should help assess travel risks and encourage both bite avoidance and chemoprophylaxis where recommended. A health belief model exploring potential barriers to chemoprophylaxis adherence and exploration of alternatives may improve counseling outcomes. While malaria prophylaxis is useful, clinicians need to educate the traveler from getting bitten by mosquitoes. Nurses should inquire about recent or upcoming travel to endemic areas, and set in motion the steps to address malaria prevention when applicable, working with the clinician to evaluate the patient. The pharmacist will have a role in vetting the agent choice as well as verifying dosing. Practitioners should take advantage of the opportunity to assess risk for the entire family and encourage all travelers to seek pretravel counseling. The key is for the clinician to educate the traveler on prevention; this means wearing appropriate garments, using DEET and mosquito nets when sleeping and not venturing out after dusk. The interprofessional team needs to coordinate efforts to execute effective malarial prophylaxis. [Level 5]
Nursing, Allied Health, and Interprofessional Team Interventions
Missed dosing of chemoprophylaxis: Typically, travelers who have missed a dose of once-weekly prophylaxis should take the medication as soon as remembered and then resume treatment on the regularly scheduled day of the week. If the patient forgets the dose for more than two days, blood levels may not be adequate. The medication should still be taken as soon as remembered, with the next dose seven days later and then weekly after that. For daily dosing regimens, the drug should be taken at the same time each day. If the dose is 1 to 2 days late, blood levels are less likely to be maintained. The patient should take any late dose as soon as they remember it, and subsequent doses should be daily at the same time of day.
Nursing, Allied Health, and Interprofessional Team Monitoring
Consider monitoring INR when patients take atovaquone-proguanil with warfarin. Assess creatinine clearance before administration of doxycycline and assess for G6PD deficiency before initiation of primaquine or tafenoquine treatment for malaria prophylaxis.