Introduction
The epidermis is comprised of 4 or 5 layers, depending on the location of the skin sampled. These layers from deep to superficial are stratum basale, stratum spinosum, stratum granulosum, stratum lucidum, and stratum corneum. The stratum lucidum is typically only present in the thick skin found in areas such as the palm or soles. The basal layer of the epidermis contains keratinocytes in their least differentiated form wherein they undergo mitosis and proliferate. As the keratinocytes mature and differentiate they migrate superficially, produce keratin, and eventually lose their nuclei and other cellular organelles; the latter cells form stratum corneum or the horny layer of the skin. Keratin, the basic constituent protein of the skin and its appendages, is arranged in the form of keratin filament bundles. Filaggrin is one of the major intermediate filament-associated proteins that interact with keratin filaments in the skin. Its precursor form called profilaggrin accumulates in cytoplasmic granules of the granular layer in the form of structures called keratohyalin granules (KHG).
Keratohyalin granules primarily exist within the stratum granulosum, with some present in the stratum spinosum. These granules are insoluble in water and located within the cytoplasm where they promote dehydration of the cell. Their chief function seems to be cross-linking of keratin filaments which creates the tight barrier that is the epidermis, providing the body with an impermeable layer that protects from invasion by foreign particles. This process of cornification of the epidermis effected by KHG is known as keratinization.
Issues of Concern
This article examines the histology of KHG found within the epidermal layer of the skin and will discuss the following: The structure and functions of KHG, tissue preparation prior to examination of granules, histochemistry and cytochemistry, electron microscopy, and relevant pathophysiology with clinical correlation.
Structure
Skin & Mucosa
Keratohyalin granules are typically present in the stratum granulosum of the epidermal layer of the skin (and epithelium of the mucosae). KHG are protein structures found in the keratinocytes of the stratum granulosum that appear in two main variants. Globular KHG are found in quickly dividing epithelia, such as the oral mucosa; whereas, stellate KHG are in the slowly dividing normal epidermis.[1] The granules typically contain a mixture of keratin filament bundles, as well as the filament-associated proteins loricrin, filaggrin, and trichohyalin.[2][3]
Thymus
Additionally, KHG is also present in the cytoplasm of Hassall’s corpuscles of the thymus gland.[4] Hassall's corpuscles, also called type VI epithelioreticular cells are present in the medulla of the thymus gland. Apart from the presence of KHG, their cytoplasm also contains intermediated fibers and may be keratinized. Although the precise function of thymic KHG and keratinization of Hassall's corpuscles remains unknown, they have been suggested to produce IL-4 and IL-7 to help with T lymphocyte development.
Function
Keratinisation
Keratohyalin granules promote the formation of the epidermal cornified cell envelope, also known as cornification or keratinization. Visualization of these granules in the various epidermal layers shows that they grow in size progressively as they move from the stratum spinosum toward the stratum corneum.[5] In the smaller keratohyalin granules located within the deeper epidermal layers, dense aggregates of ribosomes can be seen at the site of blebs, indicating protein assembly and initial keratin intermediate filament formation.[6] As keratinocytes differentiate and the granules expand in size, the keratohyalin granules convert keratin tonofilaments into a homogenous keratin matrix, an important step in cornification.[7]
The name filaggrin is a contraction of ‘filament aggregating protein.’ In vitro experiments have shown that filaggrin causes bundling and condensation of keratin intermediate filaments.[8] Various techniques including immunohistochemistry, immuno-electron microscopy, and biochemical studies have revealed that profilaggrin, the large (>400 kDa) precursor protein of filaggrin, is the main constituent of the KHG.[9] In fact, the abundance of profilaggrin/filaggrin as constituents of the KHG renders these granules visible allowing demarcation of stratum granulosum by low-power light microscopy in skin sections stained with hematoxylin & eosin.[10]
The process of keratinization, i.e., the formation of the cornified cell envelope is a complex phenomenon. It involves cross-linking of various proteins in the cell periphery, especially loricrin and involucrin at the transition zone in between stratum granulosum and stratum corneum.[11] Profilaggrin is inactive within the KHG, but during epidermal differentiation of granular layer keratinocytes committing to form the squames of stratum corneum, these granules vanish as profilaggrin rapidly processes into free filaggrin monomers.[9] This process leads to condensation of the cytoskeleton and flattening of the terminally differentiating granular cells into corneal squames. A complex cascade of phosphatases and proteases is involved in this rapid degranulation event.[9]
Epidermal Moisturisation via water retention in stratum corneum
Within the squames, filaggrin undergoes further chemical modification and proteolytic processing. Eventually, it breaks down completely to become natural moisturizing factor (NMF), a natural humectant within the residual cytoplasmic space of the squames structurally/chemically composed of hygroscopic amino acids and their derivatives.[10]
Other indirect functions
Through their contribution to keratinization and water retention in stratum corneum, KHG and its constituent proteins contribute to other functions such as UV protection, and modulation of stratum corneum pH (to make it hostile for pathogenic microbes). Thus, as a whole, KHG, filaggrin, and related proteins are vital for normal healthy function of the skin barrier layers.
Tissue Preparation
Traditionally, acetic acid has been used to obtain sheet preparations of the stratum granulosum from the epidermis and oral epithelium of rodents and man.[12] Contemporarily, a variety of effective methods of tissue preparation for the visualization of KHG have been proposed. Standard lab methods are typically appropriate for preparation and fixation of epidermal tissue. Reconstructed human epidermis (RHE) offers a recently developed model to study epidermal cellular kinetics including the KHG, lamellar bodies, and other functional structures.
Standard Method
A standard method that was found to be effective is as follows. Specimens should be immediately processed following surgical excision and washed once in 1X phosphate buffered solution. Small pieces are typically best for preparation, typically sizes less than 1 mm. Tissue can then be frozen in optimal cutting temperature medium for cryosection formation, or they can be fixed in formaldehyde or ethanol prior to paraffin embedding, after which they are sliced and placed on slides for further staining or analysis.[13][14]
Reconstructed human epidermis
Models of RHE have are useful for the evaluation of physio-chemical properties of the epidermis, metabolic studies of pharmaceutical products, epidermal responses to irritants and sensitizers, and assessment of cutaneous irritancy or phototoxicity.[15] RHE prepared by culturing normal human keratinocytes at high cell density for few weeks (average - 14 days) in a serum-free and high calcium (1.5 mM) medium on an inert polycarbonate filter at the air-liquid interface showed histological features similar to those observed in vivo in the epidermis: a proliferating basal layer and differentiating spinous, granular, and cornified layers. Electron microscopy illustrated lamellar bodies, junctions, and keratohyalin granules.[15] Such models are customizable for studies involving epidermal keratinocytes and its key constituents such as KHG, lamellar bodies, keratin intermediate filaments, etc.
Histochemistry and Cytochemistry
A variety of histochemical and immunohistochemical staining methods can be used to examine KHG in tissue samples. Several examples are listed below, which at the time of their respective publications, provided greater insight into the structure and function of keratohyalin granules.
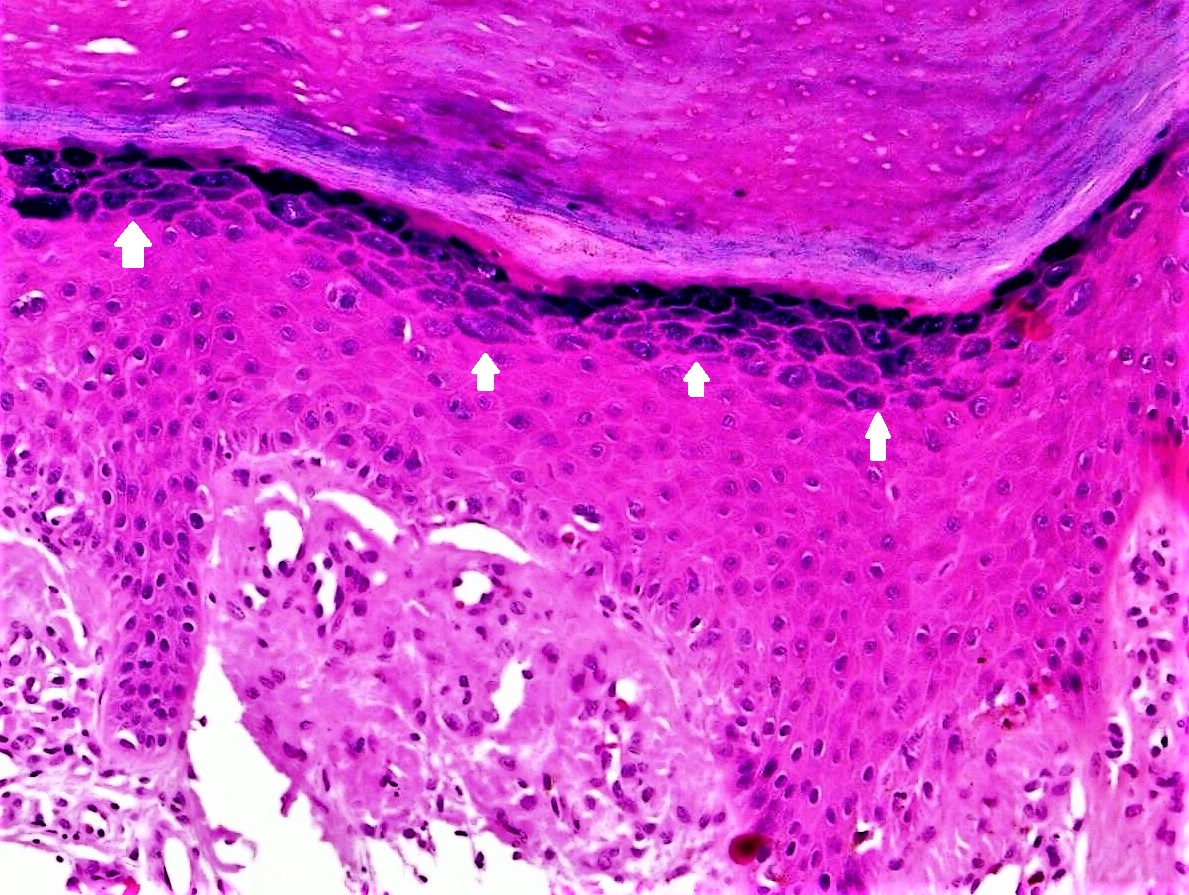
Hematoxylin and eosin (H & E) staining is a particularly effective method for examining KHG. This is in large part due to the hematoxylin stainability of the granules. The stainability of KHG is thought to be due to the fibrinogen gamma-chain protein found within the granules, which is demonstrably basophilic.[16] The basophilic staining of KHG makes them appear blue on H & E staining, which facilitates identification of stratum granulosum in the epidermis from an H & E stained section (Figure 1].
Bennett’s reagent is a simple and rapid method of identifying free sulfhydryl groups.[17] The high number of free sulfhydryl groups within KHG also makes histochemical staining with Bennett’s reagent possible. Staining shows increased density of sulfhydryl groups within the stratum granulosum, with moderate staining in the stratum spinosum.[18]
The protein filaggrin, found within KHG, has a relatively high presence of histidine. Antibodies raised against this protein have been used to immunohistochemically visualize keratohyalin granules.[19]
Microscopy, Electron
Electron microscopy has been shown to be an effective method in visualizing the process of cornification in the human skin, in which KHG play a large role. Keratohyalin granules are apparent as electron-dense granules that appear in the cytoplasm of cells where they can be seen forming a sheath around the tonofibrils and ultimately associating with keratin filaments in the more superficial layers of the epidermis.[20] Greater insight into the role of KHG in the cornification process initially came to light after treatment with proteases and under visualization by electron microscopy. The findings were that the thickening of the cell membranes in the stratum corneum is not the result of the addition of material from the outside of the cell, but instead the deposition of protein material on the cytoplasmic side of the membrane.[21]
Pathophysiology
Unique characteristics of keratohyalin granule immunohistochemistry have been used to evaluate many pathologic conditions of the skin and have led to a better understanding of healthy physiology as well. Histochemistry of the granules in human oral leukoplakia produced staining with Pauly’s reagent, Congo red, and hematoxylin, suggesting a high amount of protein within the granules; however, the keratohyalin granules did not react with Feulgen reagent, demonstrating the absence of DNA.[14]
Clinical Significance
The antiperinuclear factor is a rheumatoid arthritis-specific autoantibody that is present in 49-91% of patients with rheumatoid arthritis. Immunohistochemical staining with antibodies directed against antiperinuclear factor shows staining of KHG.[22]
Identification of hypergranulosis or hypogranulosis on H & E staining is often helpful in the histological diagnosis of skin conditions like psoriasis, lichen planus, Bowen disease, and actinic keratosis amongst others.
The presence of coarse KHG in cases of irritated seborrheic keratosis is useful to differentiate seborrheic keratosis from verruca vulgaris, as the coarse granules are a specific feature of irritated seborrheic keratosis.[23]
One of the main proteinaceous components of KHG is filaggrin, which is essential in facilitating cellular compression and the biogenesis of the stratum corneum. Loss-of-function mutations involving the filaggrin gene has been shown to be the cause of many conditions, including monogenic genodermatosis ichthyosis vulgaris and atopic dermatitis (eczema). A number of independent and complementary genetic studies demonstrate that inheritance of loss-of-function mutation in the filaggrin gene (FLG) confers a high risk of AD and related atopic diseases like ichthyosis vulgaris.[10]