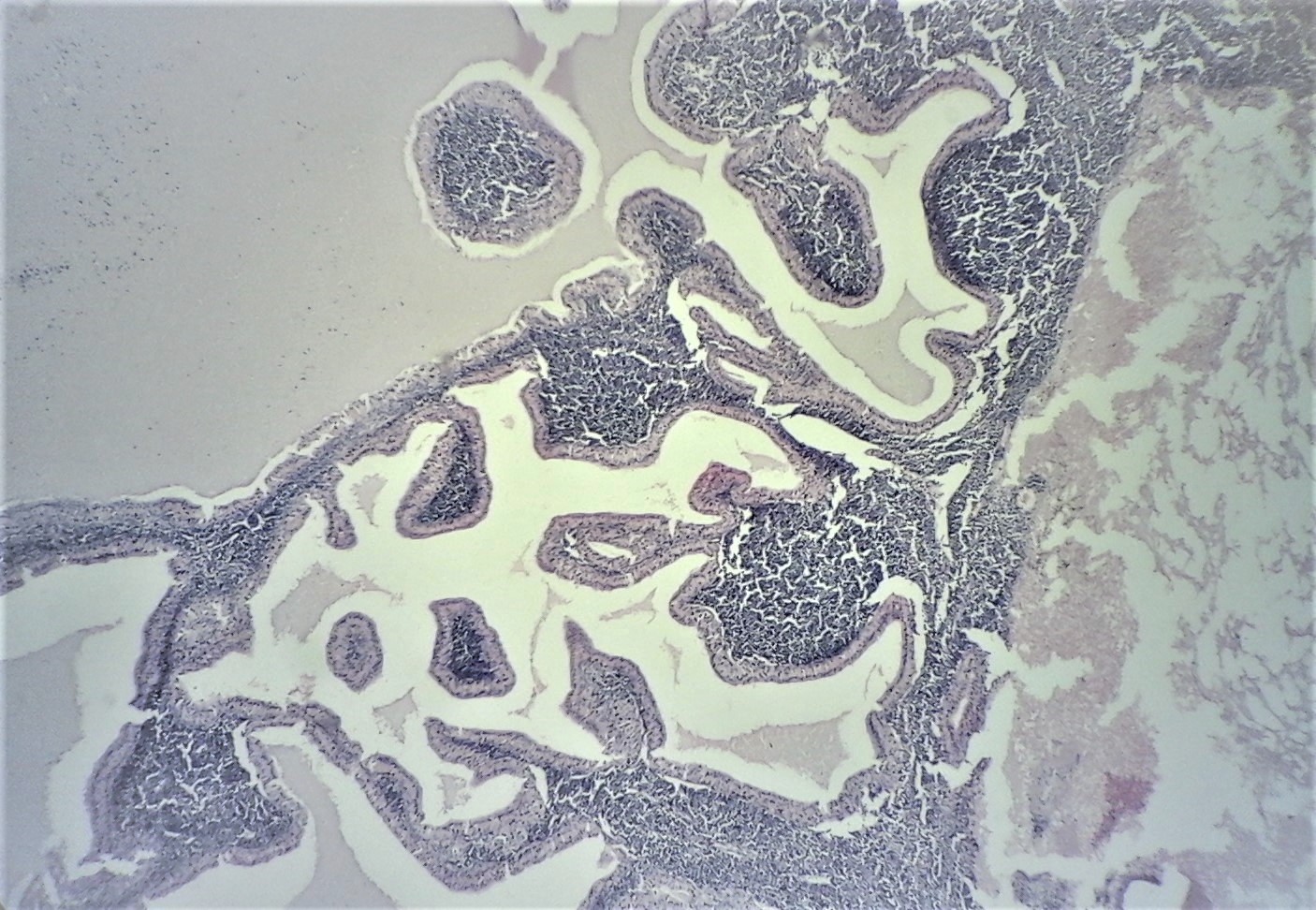
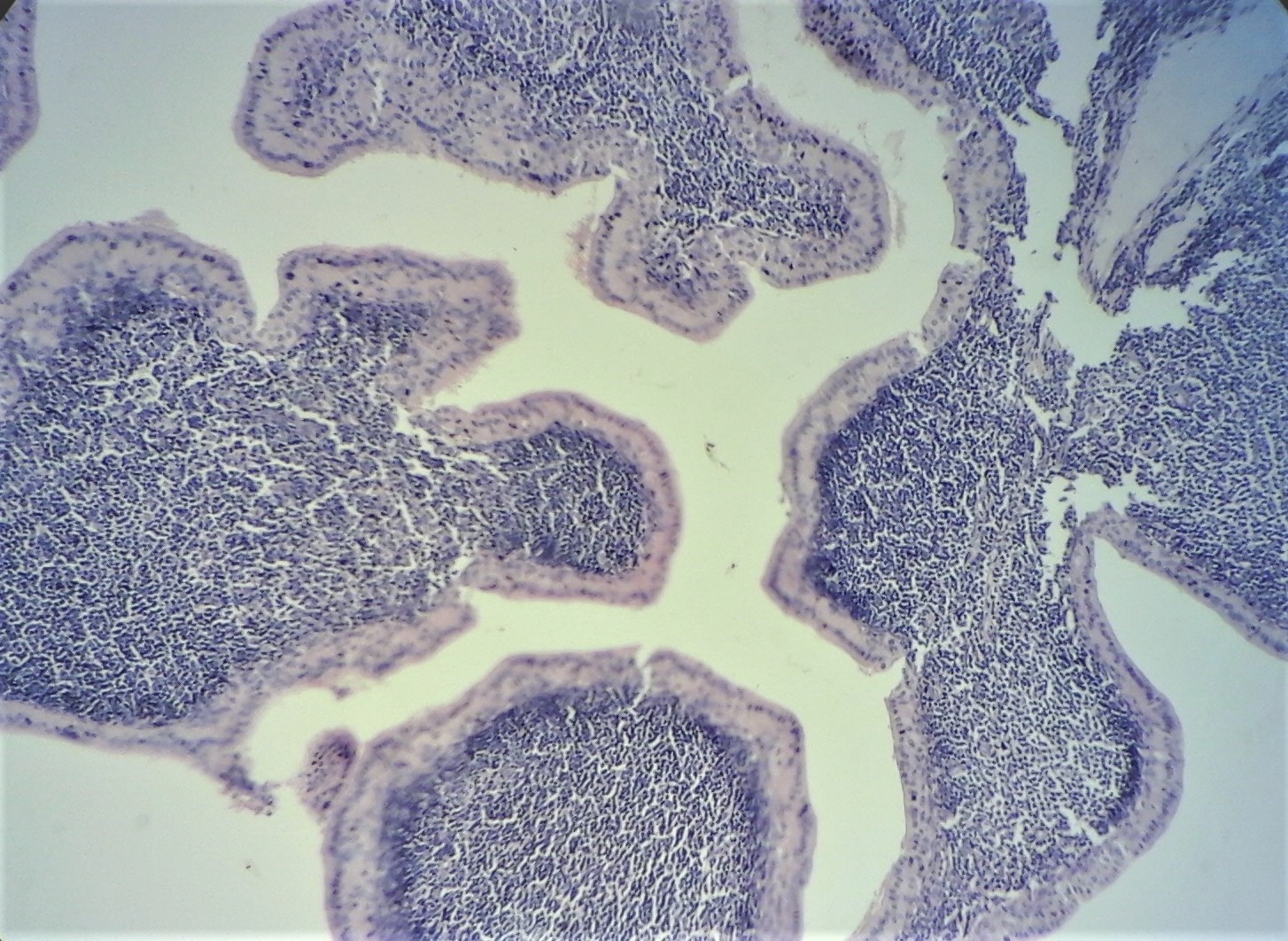
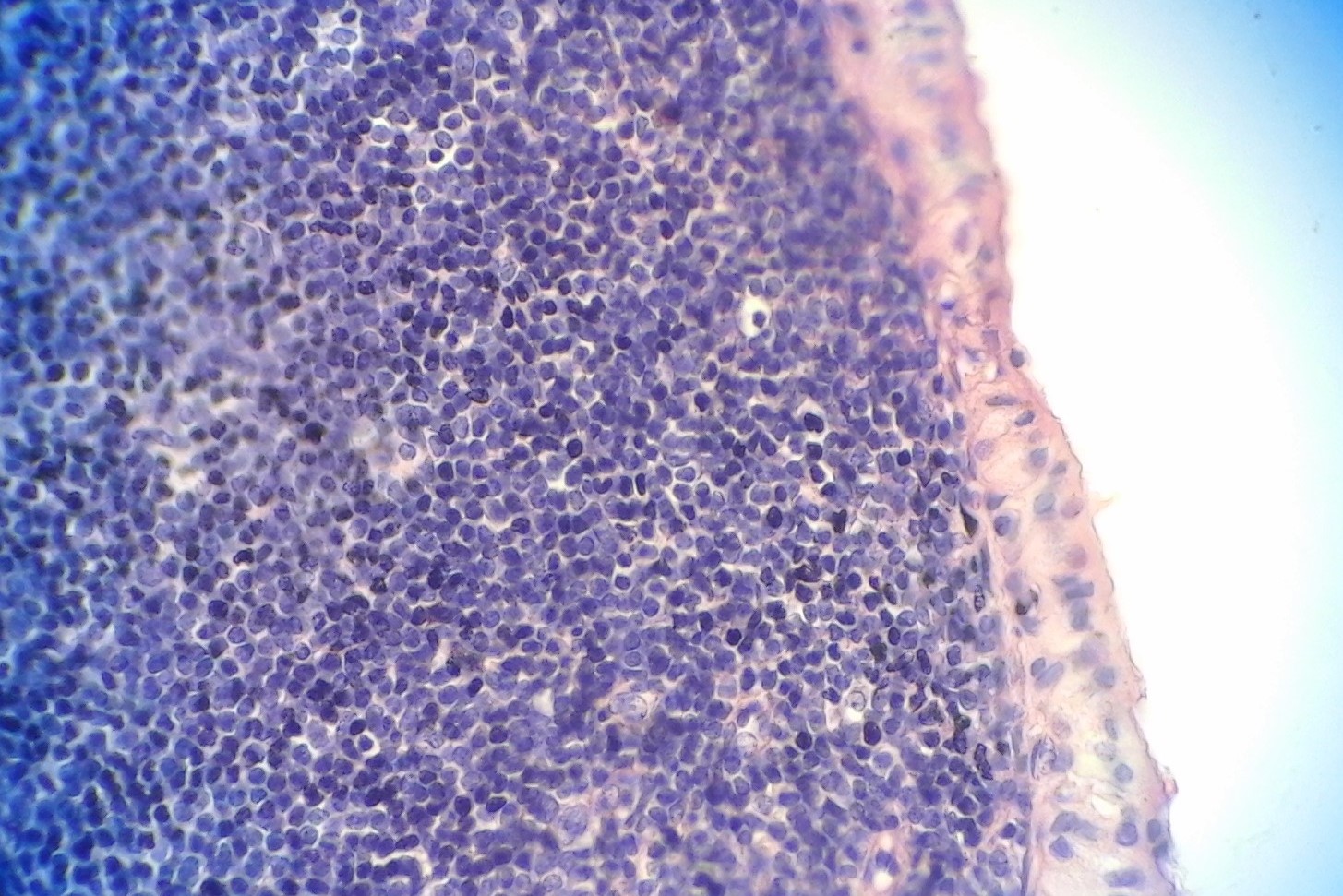
[1]
Mathur A, Mehrotra ML, Dhaliwal U. Warthin's tumour of the eye lid. Indian journal of ophthalmology. 1989 Oct-Dec:37(4):193
[PubMed PMID: 2638310]
[2]
Maiorano E, Lo Muzio L, Favia G, Piattelli A. Warthin's tumour: a study of 78 cases with emphasis on bilaterality, multifocality and association with other malignancies. Oral oncology. 2002 Jan:38(1):35-40
[PubMed PMID: 11755819]
Level 3 (low-level) evidence
[3]
Gallo O, Bocciolini C. Warthin's tumour associated with autoimmune diseases and tobacco use. Acta oto-laryngologica. 1997 Jul:117(4):623-7
[PubMed PMID: 9288224]
[4]
Cennamo A, Falsetto A, Gallo G, Lanna M, Calleri G, Di Giacomo D. Warthin's tumour in the parotid gland (an inflammatory or a neoplastic disease?). Chirurgia italiana. 2000 Jul-Aug:52(4):361-7
[PubMed PMID: 11190526]
[5]
Kristensen S, Tveterås K, Friedmann I, Thomsen P. Nasopharyngeal Warthin's tumour: a metaplastic lesion. The Journal of laryngology and otology. 1989 Jun:103(6):616-9
[PubMed PMID: 2769033]
[6]
Kale AD, Prasad UC. Re: Yu et al. Smoking and the development of Warthin's tumour of the parotid gland. The British journal of oral & maxillofacial surgery. 1999 Jun:37(3):245
[PubMed PMID: 10454034]
[7]
Therkildsen MH, Christensen N, Andersen LJ, Larsen S, Katholm M. Malignant Warthin's tumour: a case study. Histopathology. 1992 Aug:21(2):167-71
[PubMed PMID: 1505934]
Level 3 (low-level) evidence
[8]
Nishikawa H, Kirkham N, Hogbin BM. Synchronous extra-parotid Warthin's tumour. The Journal of laryngology and otology. 1989 Aug:103(8):792-3
[PubMed PMID: 2769053]
[9]
A R R, Rehani S, Bishen KA, Sagari S. Warthin's Tumour: A Case Report and Review on Pathogenesis and its Histological Subtypes. Journal of clinical and diagnostic research : JCDR. 2014 Sep:8(9):ZD37-40. doi: 10.7860/JCDR/2014/8503.4908. Epub 2014 Sep 20
[PubMed PMID: 25386545]
Level 3 (low-level) evidence
[10]
Kotwall CA. Smoking as an etiologic factor in the development of Warthin's tumor of the parotid gland. American journal of surgery. 1992 Dec:164(6):646-7
[PubMed PMID: 1334381]
[11]
THOMPSON AS, BRYANT HC Jr. Histogenesis of papillary cystadenoma lymphomatosum (Warthin's tumor) of the parotid salivary gland. The American journal of pathology. 1950 Sep:26(5):807-49
[PubMed PMID: 15432614]
[12]
Bullerdiek J, Haubrich J, Meyer K, Bartnitzke S. Translocation t(11;19)(q21;p13.1) as the sole chromosome abnormality in a cystadenolymphoma (Warthin's tumor) of the parotid gland. Cancer genetics and cytogenetics. 1988 Oct 1:35(1):129-32
[PubMed PMID: 3180001]
[13]
Aguirre JM, Echebarría MA, Martínez-Conde R, Rodriguez C, Burgos JJ, Rivera JM. Warthin tumor. A new hypothesis concerning its development. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1998 Jan:85(1):60-3
[PubMed PMID: 9474616]
[14]
Seifert G, Bull HG, Donath K. Histologic subclassification of the cystadenolymphoma of the parotid gland. Analysis of 275 cases. Virchows Archiv. A, Pathological anatomy and histology. 1980:388(1):13-38
[PubMed PMID: 7467121]
Level 3 (low-level) evidence
[15]
Santucci M, Gallo O, Calzolari A, Bondi R. Detection of Epstein-Barr viral genome in tumor cells of Warthin's tumor of parotid gland. American journal of clinical pathology. 1993 Dec:100(6):662-5
[PubMed PMID: 8249914]
[16]
Köybaşioğlu FF, Önal B, Han Ü, Adabağ A, Şahpaz A. Cytomorphological findings in diagnosis of Warthin tumor. Turkish journal of medical sciences. 2020 Feb 13:50(1):148-154. doi: 10.3906/sag-1901-215. Epub 2020 Feb 13
[PubMed PMID: 31769640]
[17]
Schindler S, Nayar R, Dutra J, Bedrossian CW. Diagnostic challenges in aspiration cytology of the salivary glands. Seminars in diagnostic pathology. 2001 May:18(2):124-46
[PubMed PMID: 11403256]
[18]
Kawasaki T, Kanamaru T, Shingaki S, Mizutani H, Nakajima T. Cytologic study of salivary gland tumors. International journal of oral surgery. 1980 Feb:9(1):68-73
[PubMed PMID: 6769832]
[19]
Chen SL, Hwang CC, Liu YC, Chen WT, Yang SW. Warthin's tumor with necrotizing tuberculous granulomatous inflammation causing severe facial nerve adhesion in parotid gland: A case report and literature review. Medicine. 2020 Feb:99(7):e18763. doi: 10.1097/MD.0000000000018763. Epub
[PubMed PMID: 32049782]
Level 3 (low-level) evidence
[20]
Psychogios G, Vlastos I, Thölken R, Zenk J. Warthin's tumour seems to be the most common benign neoplasm of the parotid gland in Germany. European archives of oto-rhino-laryngology : official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS) : affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2020 Jul:277(7):2081-2084. doi: 10.1007/s00405-020-05894-z. Epub 2020 Mar 18
[PubMed PMID: 32189070]
[21]
Párraga-Linares L, Aguirre-Urízar JM, Berini-Aytés L, Gay-Escoda C. Papillary cystoadenoma lymphomatosum (Warthin-like) of minor salivary glands. Medicina oral, patologia oral y cirugia bucal. 2009 Nov 1:14(11):e597-600
[PubMed PMID: 19680204]
[22]
Shimizu M, Ussmüller J, Hartwein J, Donath K. A comparative study of sonographic and histopathologic findings of tumorous lesions in the parotid gland. Oral surgery, oral medicine, oral pathology, oral radiology, and endodontics. 1999 Dec:88(6):723-37
[PubMed PMID: 10625857]
Level 2 (mid-level) evidence
[23]
Alibek S, Zenk J, Bozzato A, Lell M, Grunewald M, Anders K, Rabe C, Iro H, Bautz W, Greess H. The value of dynamic MRI studies in parotid tumors. Academic radiology. 2007 Jun:14(6):701-10
[PubMed PMID: 17502260]
[24]
Batsakis JG. Carcinoma ex papillary cystadenoma lymphomatosum. Malignant Warthin's tumor. The Annals of otology, rhinology, and laryngology. 1987 Mar-Apr:96(2 Pt 1):234-5
[PubMed PMID: 3566067]
[25]
Teymoortash A, Krasnewicz Y, Werner JA. Clinical features of cystadenolymphoma (Warthin's tumor) of the parotid gland: a retrospective comparative study of 96 cases. Oral oncology. 2006 Jul:42(6):569-73
[PubMed PMID: 16469528]
Level 2 (mid-level) evidence
[26]
McLean-Holden AC, Bishop JA. Low Molecular Weight Cytokeratin Immunohistochemistry Reveals That Most Salivary Gland Warthin Tumors and Lymphadenomas Arise in Intraparotid Lymph Nodes. Head and neck pathology. 2021 Jun:15(2):438-442. doi: 10.1007/s12105-020-01215-2. Epub 2020 Aug 31
[PubMed PMID: 32865726]
[27]
Kurian EM, Miller R, Mclean-Holden AL, Oliai BR, Bishop JA. Low Molecular Weight Cytokeratin Immunostaining for Extrafollicular Reticulum Cells is an Effective Means of Separating Salivary Gland Tumor-Associated Lymphoid Proliferation from True Lymph Node Involvement. Head and neck pathology. 2020 Sep:14(3):593-597. doi: 10.1007/s12105-019-01080-8. Epub 2019 Sep 20
[PubMed PMID: 31541432]