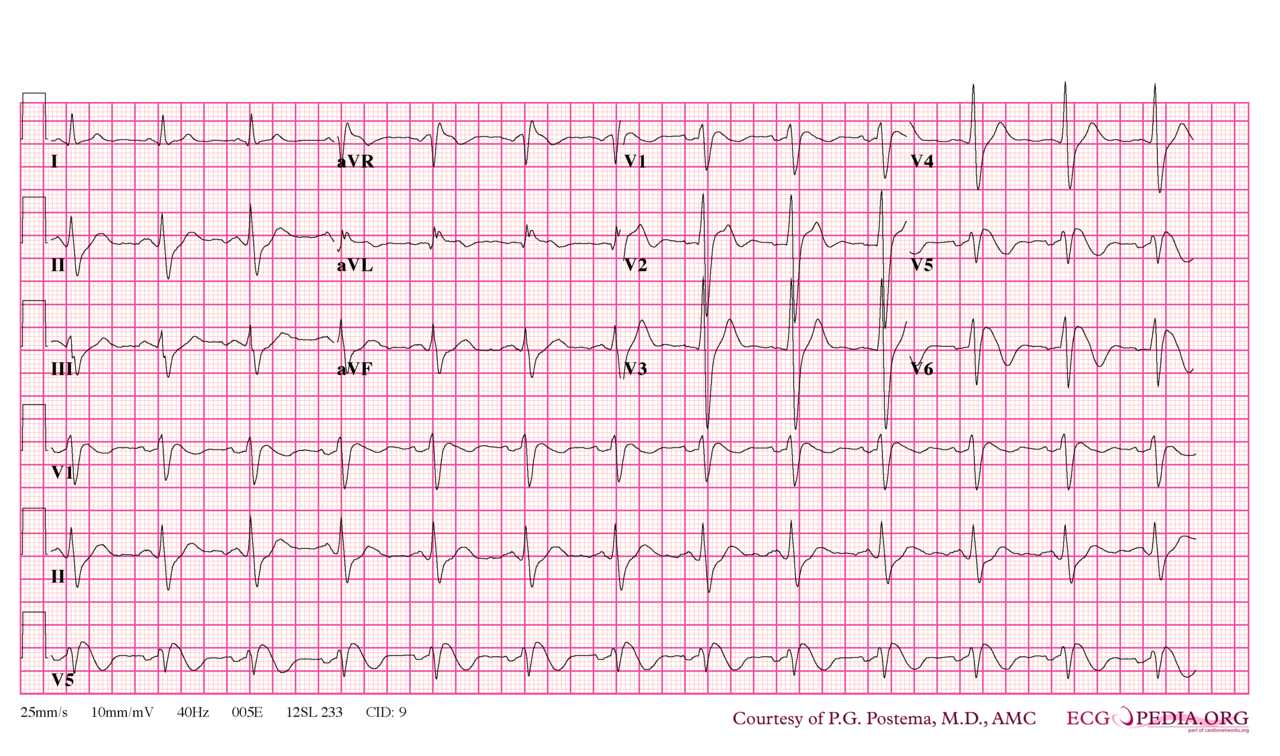
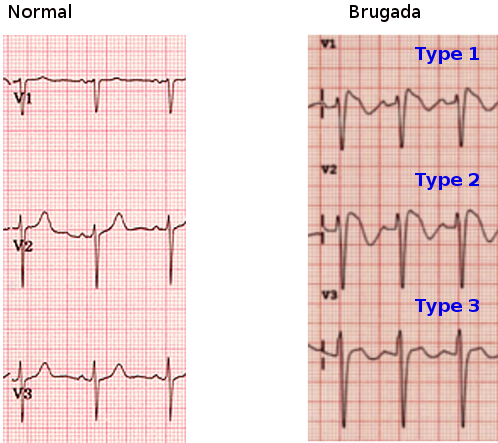
[1]
Brugada P, Brugada J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report. Journal of the American College of Cardiology. 1992 Nov 15:20(6):1391-6
[PubMed PMID: 1309182]
[2]
Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris-Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze-Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AA, Brugada R, Schott JJ, Ackerman MJ. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart rhythm. 2010 Jan:7(1):33-46. doi: 10.1016/j.hrthm.2009.09.069. Epub 2009 Oct 8
[PubMed PMID: 20129283]
[3]
Sarquella-Brugada G, Campuzano O, Arbelo E, Brugada J, Brugada R. Brugada syndrome: clinical and genetic findings. Genetics in medicine : official journal of the American College of Medical Genetics. 2016 Jan:18(1):3-12. doi: 10.1038/gim.2015.35. Epub 2015 Apr 23
[PubMed PMID: 25905440]
[4]
Meregalli PG,Wilde AA,Tan HL, Pathophysiological mechanisms of Brugada syndrome: depolarization disorder, repolarization disorder, or more? Cardiovascular research. 2005 Aug 15
[PubMed PMID: 15913579]
[5]
Littmann L, Monroe MH, Kerns WP 2nd, Svenson RH, Gallagher JJ. Brugada syndrome and "Brugada sign": clinical spectrum with a guide for the clinician. American heart journal. 2003 May:145(5):768-78
[PubMed PMID: 12766732]
[6]
Sieira J, Dendramis G, Brugada P. Pathogenesis and management of Brugada syndrome. Nature reviews. Cardiology. 2016 Dec:13(12):744-756. doi: 10.1038/nrcardio.2016.143. Epub 2016 Sep 15
[PubMed PMID: 27629507]
[7]
Louis C, Calamaro E, Vinocur JM. Hereditary arrhythmias and cardiomyopathies: decision-making about genetic testing. Current opinion in cardiology. 2018 Jan:33(1):78-86. doi: 10.1097/HCO.0000000000000477. Epub
[PubMed PMID: 29059074]
Level 3 (low-level) evidence
[8]
Mizusawa Y, Recent advances in genetic testing and counseling for inherited arrhythmias. Journal of arrhythmia. 2016 Oct
[PubMed PMID: 27761163]
Level 3 (low-level) evidence