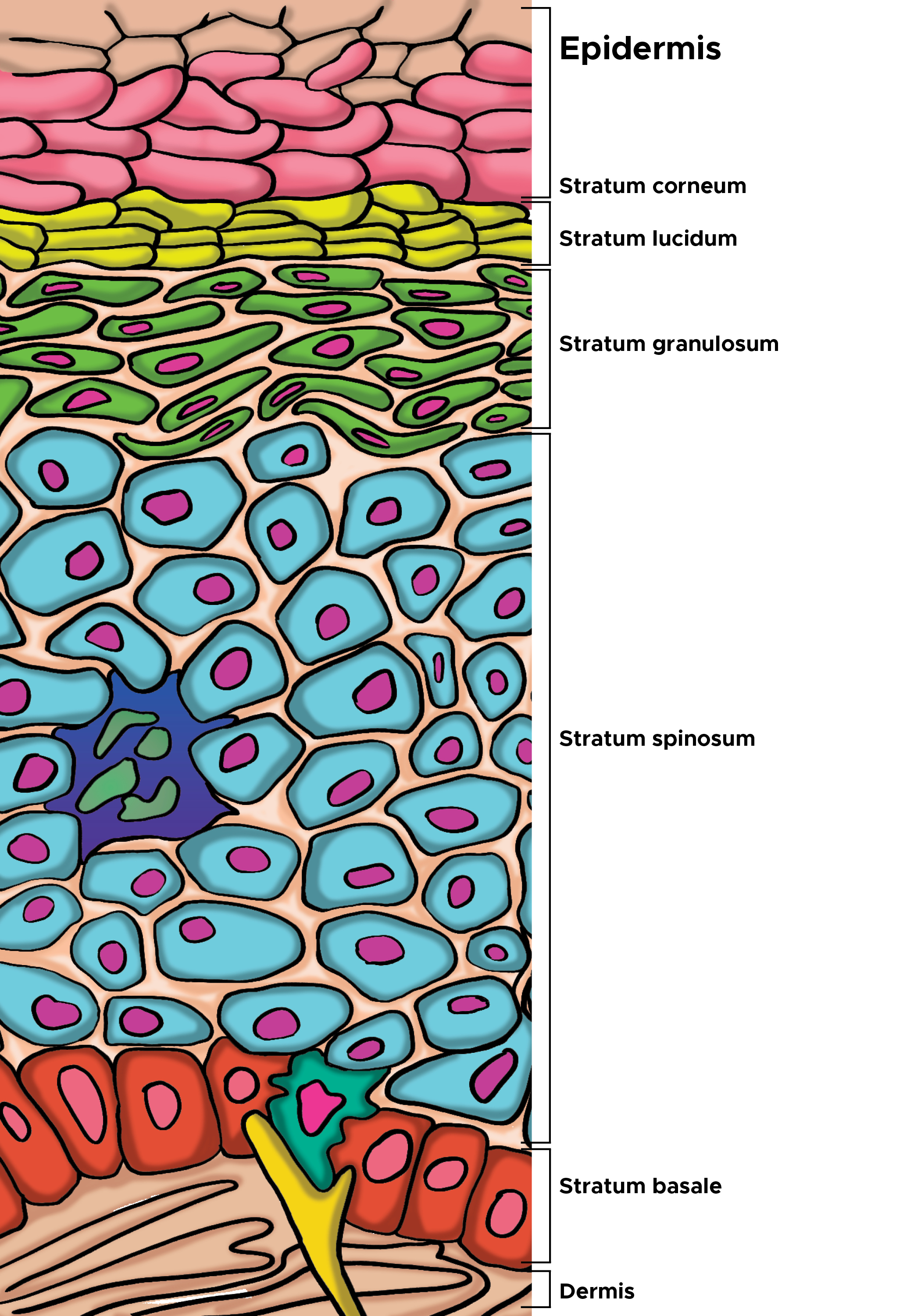
[2]
Green EM, Mansfield JC, Bell JS, Winlove CP. The structure and micromechanics of elastic tissue. Interface focus. 2014 Apr 6:4(2):20130058. doi: 10.1098/rsfs.2013.0058. Epub
[PubMed PMID: 24748954]
[3]
Cotta-Pereira G, Guerra Rodrigo F, Bittencourt-Sampaio S. Oxytalan, elaunin, and elastic fibers in the human skin. The Journal of investigative dermatology. 1976 Mar:66(3):143-8
[PubMed PMID: 1249442]
[4]
Uitto J,Li Q,Urban Z, The complexity of elastic fibre biogenesis in the skin--a perspective to the clinical heterogeneity of cutis laxa. Experimental dermatology. 2013 Feb
[PubMed PMID: 23088642]
Level 3 (low-level) evidence
[5]
Prost-Squarcioni C, Fraitag S, Heller M, Boehm N. [Functional histology of dermis]. Annales de dermatologie et de venereologie. 2008 Jan:135(1 Pt 2):1S5-20. doi: 10.1016/S0151-9638(08)70206-0. Epub
[PubMed PMID: 18442658]
[6]
Hashmi S, Marinkovich MP. Molecular organization of the basement membrane zone. Clinics in dermatology. 2011 Jul-Aug:29(4):398-411. doi: 10.1016/j.clindermatol.2011.01.009. Epub
[PubMed PMID: 21679867]
[7]
Sethu C, Sethu AU. Glomus tumour. Annals of the Royal College of Surgeons of England. 2016 Jan:98(1):e1-2. doi: 10.1308/rcsann.2016.0005. Epub
[PubMed PMID: 26688416]
[8]
Friske JE,Sharma V,Kolpin SA,Webber NP, Extradigital glomus tumor: a rare etiology for wrist soft tissue mass. Radiology case reports. 2016 Sep
[PubMed PMID: 27594949]
Level 3 (low-level) evidence
[9]
Cui CY, Schlessinger D. Eccrine sweat gland development and sweat secretion. Experimental dermatology. 2015 Sep:24(9):644-50. doi: 10.1111/exd.12773. Epub 2015 Jul 14
[PubMed PMID: 26014472]
[10]
Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science (New York, N.Y.). 2014 Nov 21:346(6212):950-4. doi: 10.1126/science.1254229. Epub
[PubMed PMID: 25414303]
[11]
Wilgus TA, Wulff BC. The Importance of Mast Cells in Dermal Scarring. Advances in wound care. 2014 Apr 1:3(4):356-365
[PubMed PMID: 24757590]
Level 3 (low-level) evidence
[12]
Kruglikov IL,Scherer PE, Dermal Adipocytes: From Irrelevance to Metabolic Targets? Trends in endocrinology and metabolism: TEM. 2016 Jan
[PubMed PMID: 26643658]
[13]
Driskell RR, Jahoda CA, Chuong CM, Watt FM, Horsley V. Defining dermal adipose tissue. Experimental dermatology. 2014 Sep:23(9):629-31. doi: 10.1111/exd.12450. Epub
[PubMed PMID: 24841073]
[14]
Jindal A, Rao R, Bhogal BS. Advanced Diagnostic Techniques in Autoimmune Bullous Diseases. Indian journal of dermatology. 2017 May-Jun:62(3):268-278. doi: 10.4103/ijd.IJD_196_17. Epub
[PubMed PMID: 28584369]
[15]
Lee DY, Kim YJ, Lee JY, Kim MK, Yoon TY. Primary localized cutaneous nodular amyloidosis following local trauma. Annals of dermatology. 2011 Nov:23(4):515-8. doi: 10.5021/ad.2011.23.4.515. Epub 2011 Nov 3
[PubMed PMID: 22148024]
[16]
Gaviria JL,Ortega VG,Gaona J,Motta A,Medina Barragán OJ, Unusual Dermatological Manifestations of Gout: Review of Literature and a Case Report. Plastic and reconstructive surgery. Global open. 2015 Jul
[PubMed PMID: 26301134]
Level 3 (low-level) evidence
[17]
Yurchenco PD, Patton BL. Developmental and pathogenic mechanisms of basement membrane assembly. Current pharmaceutical design. 2009:15(12):1277-94
[PubMed PMID: 19355968]
[18]
Hansen B, Jemec GB. The mechanical properties of skin in osteogenesis imperfecta. Archives of dermatology. 2002 Jul:138(7):909-11
[PubMed PMID: 12071818]
[19]
Pepe G, Giusti B, Sticchi E, Abbate R, Gensini GF, Nistri S. Marfan syndrome: current perspectives. The application of clinical genetics. 2016:9():55-65. doi: 10.2147/TACG.S96233. Epub 2016 May 9
[PubMed PMID: 27274304]
Level 3 (low-level) evidence
[21]
Ud-Din S,McGeorge D,Bayat A, Topical management of striae distensae (stretch marks): prevention and therapy of striae rubrae and albae. Journal of the European Academy of Dermatology and Venereology : JEADV. 2016 Feb
[PubMed PMID: 26486318]
[22]
Baum J,Duffy HS, Fibroblasts and myofibroblasts: what are we talking about? Journal of cardiovascular pharmacology. 2011 Apr
[PubMed PMID: 21297493]
[23]
Lee JY, Yang CC, Chao SC, Wong TW. Histopathological differential diagnosis of keloid and hypertrophic scar. The American Journal of dermatopathology. 2004 Oct:26(5):379-84
[PubMed PMID: 15365369]
[24]
Jumper N,Paus R,Bayat A, Functional histopathology of keloid disease. Histology and histopathology. 2015 Sep
[PubMed PMID: 25900252]
[25]
Nair PA. Vulvar Lichen Sclerosus et Atrophicus. Journal of mid-life health. 2017 Apr-Jun:8(2):55-62. doi: 10.4103/jmh.JMH_13_17. Epub
[PubMed PMID: 28706405]
[26]
Puri N, A study of pathogenesis of acanthosis nigricans and its clinical implications. Indian journal of dermatology. 2011 Nov
[PubMed PMID: 22345770]
[28]
Piera-Velazquez S,Louneva N,Fertala J,Wermuth PJ,Del Galdo F,Jimenez SA, Persistent activation of dermal fibroblasts from patients with gadolinium-associated nephrogenic systemic fibrosis. Annals of the rheumatic diseases. 2010 Nov
[PubMed PMID: 20570839]
[29]
Heng JK,Aw DC,Tan KB, Solar elastosis in its papular form: uncommon, mistakable. Case reports in dermatology. 2014 Jan
[PubMed PMID: 24926253]
Level 3 (low-level) evidence
[30]
Ceilley RI. Treatment of Actinic Purpura. The Journal of clinical and aesthetic dermatology. 2017 Jun:10(6):44-50
[PubMed PMID: 28979656]
[31]
Mravic M, LaChaud G, Nguyen A, Scott MA, Dry SM, James AW. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. International journal of surgical pathology. 2015 May:23(3):181-8. doi: 10.1177/1066896914567330. Epub 2015 Jan 22
[PubMed PMID: 25614464]
Level 3 (low-level) evidence
[34]
Bhat RM,Prakash C, Leprosy: an overview of pathophysiology. Interdisciplinary perspectives on infectious diseases. 2012
[PubMed PMID: 22988457]
Level 3 (low-level) evidence
[36]
Soto R,Levy Y,Krause JR, Sweet syndrome and its association with hematopoietic neoplasms. Proceedings (Baylor University. Medical Center). 2015 Jan
[PubMed PMID: 25552802]
[37]
Nair PA,Singhal R, Xanthelasma palpebrarum - a brief review. Clinical, cosmetic and investigational dermatology. 2018
[PubMed PMID: 29296091]
[39]
Bhattacharya S,Mishra RK, Pressure ulcers: Current understanding and newer modalities of treatment. Indian journal of plastic surgery : official publication of the Association of Plastic Surgeons of India. 2015 Jan-Apr
[PubMed PMID: 25991879]
Level 3 (low-level) evidence
[40]
Touart DM, Sau P. Cutaneous deposition diseases. Part I. Journal of the American Academy of Dermatology. 1998 Aug:39(2 Pt 1):149-71; quiz 172-4
[PubMed PMID: 9704823]